All published articles of this journal are available on ScienceDirect.
Outcomes of Patients with Cardiac Myxoma: A Retrospective Multicentre Study
Abstract
Background:
We present a 15-year follow-up of patients with Cardiac Myxoma (CM) who underwent tumour resection.
Patients and Methods:
Between 2001 and 2016, 38 patients with CM were operated on. We retrospectively reviewed, their clinical presentations. We also analysed the echocardiographic, electrocardiographic and pathology reports.
Results:
No mortality was seen after surgery. The mean age of the patients was 41.7±7.8 years and the female/male ratio was 22/16. Two patients with CM were children. The main symptoms of left atrial CM were dyspnoea on exertion, chest pain and congestive heart failure. Tricuspid valve pathology, and leg oedema were the main symptoms in patients with right atrial CM combined with right heart failure. CM as a cause of nephrotic syndrome was detected in 2 patients. In 5 asymptomatic patients (13.1%), CM was detected incidentally. We detected a pericardial invasion by the tumour in 1 patient. We performed secondary surgery because of tumour recurrence in 2 patients. Overall, survival for patients after surgical excision was 96.4±1.6% at 1 year, 91.7±2.4% at 5 years, 87.6±2.6% at 10 years and 85±1.9% at 15 years.
Conclusion:
The symptoms of CM may include congestive heart failure or nephrotic syndrome. Because CM may be associated with serious cardiac symptoms, tumour excision should be performed immediately after diagnosis. The use of right anterior thoracotomy for CM resection is a safe surgical approach.
1. INTRODUCTION
A Cardiac Myxoma (CM) is the most common type of primary cardiac tumour [1]. Approximately more than half of primary cardiac tumours are myxomas [2-5]. They are most commonly diagnosed between the age of 30 and 60 years [5]. CM is a sporadic or familial disorder [5, 6].
A limited number of patients are referred with the classical triad of obstructive cardiac symptoms: pulmonary oedema, progressive heart failure (CHF) or arterial embolic events [6, 7]. Rarely, syncope/vertigo or sudden death can be the first symptom of a CM [6-9].
For early diagnosis, transthoracic echocardiography (TTE) is increasingly used. Magnetic resonance imaging (MRI) and/or thoracic Computed Tomography (CT) have also been used. Early and optimal surgical excision have shown excellent early and long-term results, with no recurrence of the tumour [10-12].
According to previous publications [13-16] CMs are diagnosed sporadically in 90% of patients. In contrast to solid CMs, papillary CMs are characterized by a soft formation which is friable during the excision. Therefore, the rate of recurrence is higher in patients with papillary CMs compared with the solid type.
We present our experience over the past 15 years of 38 patients with CMs with unusual side effects such as renal failure (which required haemodialysis), nephrotic syndrome and pericardial invasion.
2. PATIENTS AND METHODS
The clinical outcomes of 38 patients with cardiac CMs who were treated surgically between June 2001 and January 2016 were retrospectively analysed. An institutional ethics committee approved this study. Patient characteristics and symptoms are listed in Table 1. The mean age of the patients was 41.7±7.8 years and the female/male ratio was 22/16. The mean duration between symptoms and diagnosis was 5.7±0.3 months (range: 17 days - 8.3 months). The tumours were diagnosed incidentally using a TTE as part of a check-up programme in 5 asymptomatic patients (13.1%). Three patients were referred to our emergency unit because of an acute arterial embolic event. Two patients who had a cerebrovascular event were followed in the ICU by a neurologist and anaesthetist prior to surgery.
Two patients with CM were 13 and 17 years old; 11 patients had chest pain and respiratory discomfort on exertion; 7 patients had a history of early fatigue during walking. Non-specific findings such as anaemia, weight loss, cough, and fever were detected in 6 patients. None of the patients had a family history or Carney complex [12-17] which is characterized by spotty skin pigmentation, endocrine overactivity and myxomas [15-18]. Pathological classification showed solid (sessile) CMs in 18 patients (47.3%); papillary CMs were detected in the other 20 patients. The most common site was the left atrium. Left atrial and interatrial septal defect were common. Classification of the patients according to tumour implantation area, medication, and the risk factors such as tumour size are summarised in Table 2.
| Male/Female | 16/22 |
|---|---|
| Age (mean ± SD, years) | 41±7.8 |
| BMI (mean ± SD) | 23.1±2.6 |
| Left Atrial | 81.6% |
| Right Atrial | 18.4% |
| Symptoms (n) | |
| Incidental | 5 |
| Dyspnoea | 11 |
| Chest pain | 14 |
| CHF | 7 |
| Palpitation | 2 |
| CVA | 3 |
| Systemic arterial embolus | 2 |
| Renal failure | 2 |
| Constitutional Symptom (n) | |
| Weight loss | 6 |
| Vertigo/Syncope | 3 |
| Early fatigue | 7 |
| Limb oedema | 8 |
| NYHA Classification (n) | |
| Class-I | 14 |
| Class-II | 10 |
| Class-III | 9 |
| Class-IV | 5 |
| Origin Site | n of Patients |
|---|---|
| Left atrium | 26 |
| Interatrial septum | 3 |
| Left atrial wall | 1 |
| Posterior mitral leaflet | 4 |
| Right atrium | 4 |
According to the New York Heart Association (NYHA) functional class: 14 patients were NYHA Class I, 10 patients were NYHA Class-II and 9 patients were class III. The remaining 5 patients were class IV (Table 1). All patients showed a high sedimentation rate. Electrocardiograms demonstrated sinus tachycardia in 2 patients and paroxysmal Atrial Fibrillation (AF) in 4 patients. Non-specific ST segment abnormalities were observed in 6 patients. The mean ejection fraction was 52±4.9% by echocardiography. Serious valvular heart disease and coronary artery stenosis requiring additional surgery were detected in 7 patients. Additional cardiac surgery concomitant with CM resection, and an age distribution of our patients are summarized in Tables 3 and 4.
| n of patients | |
|---|---|
| MV Repair | 2 |
| AV Repair | 1 |
| TV Repair | 2 |
| CABG | 2 |
| Age (years) | n of Patients |
|---|---|
| 14–20 | 4 |
| 21–35 | 6 |
| 36–40 | 11 |
| 41–64 | 17 |
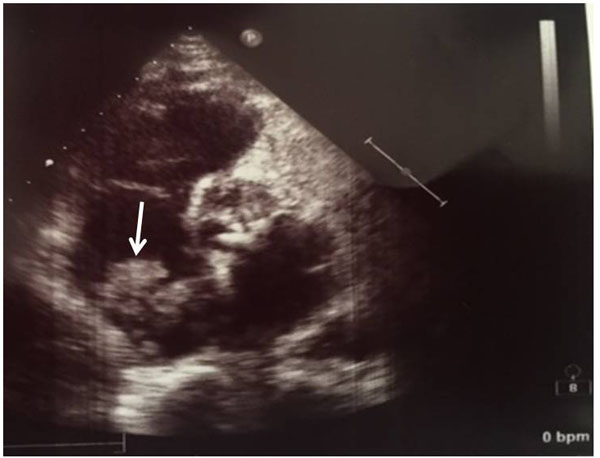
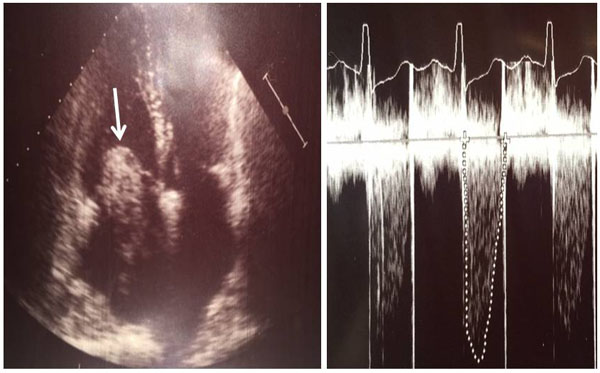
We used two-dimensional TTE for diagnosis in all patients (Figs. 1 and 2). If tumours other than CMs were suspected, thoracic CT or MRI were used. Coronary angiography was performed routinely in patients with a history of chest pain or older than 45 years. Symptomatic cardiac arrhythmias were described as: non-sustained and sustained ventricular tachycardia, sustained supraventricular tachycardia (atrial flutter or AF) and a frequent ventricular ectopic beats. Early post-operative mortality was defined as death in hospital or within 30 days after surgery. After discharge, physical examinations, electrocardiograms, chest radiographs, and echocardiograms were performed at 6 months, and thereafter every year. If we suspected a recurrence, we performed a TTE and/or MRI.
Two previously healthy patients had been referred to general practitioners with a history of bilateral leg swelling and fatigue. Investigations showed proteinuria which prompted referral to a nephrologist. Formal urinalysis confirmed proteinuria (545 mg/dL). Renal biopsy was performed in another hospital. Because there was no aetiology, echocardiography was performed. Renal replacement therapy was performed prior to excision of CMs. Three days after surgery, proteinuria gradually decreased; Blood Urea Nitrogen (BUN) and creatinine levels were nearly within normal limits. These patients were discharged and a urinary protein concentration of 7-11 mg/dL confirmed the resolution of proteinuria.
2.1. Surgical Technique
We used a median sternotomy with extracorporeal circulation in 20 patients. In patients with isolated CM, surgery was performed via right anterior thoracotomy incision. Mild or moderate hypothermia was used. To protect from the risk of tumour fragmentation or embolization, handling of the heart was not performed before aortic cross-clamping. We prefer a right or left atrial approach or biatrial technique for CM resection. Tumours were totally excised, as well as an adjacent cuff of endocardium surrounding the attachment of the CMs. We irrigated the cardiac chambers including ventricles using with saline to remove any tumour fragments after excision.
Low Cardiac Out-Put Syndrome (LCOS) developed in 3 patients. Intraoperative TEE was performed in order to avoid the possibility of additional CMs in other cardiac chambers and post-surgery control of valvular repair or replacement.
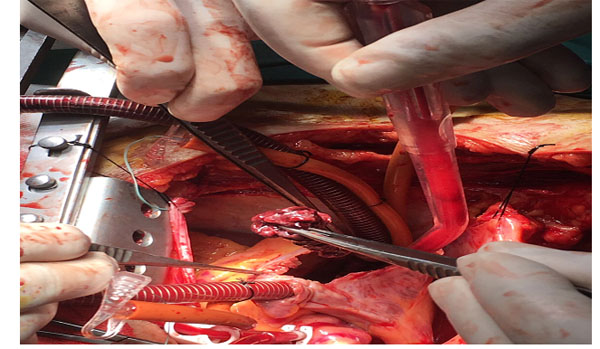
Mitral, tricuspid or aortic valve repair were performed in 5 patients. De Vega annuloplasty or Key Annuloplasty was performed (Figs. 3a and b). In 2 patients with atrial CM concomitant with coronary artery disease, we performed coronary artery bypass grafting.
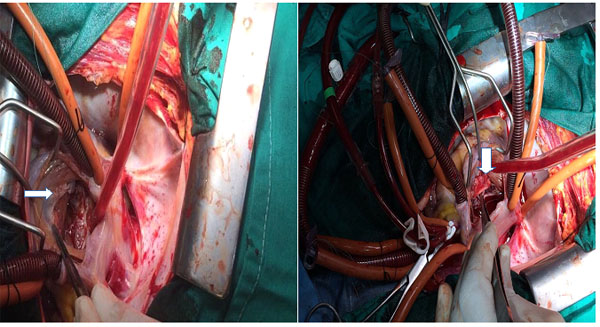
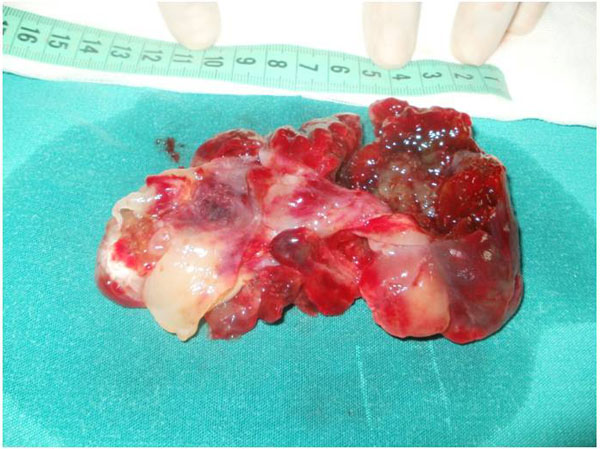
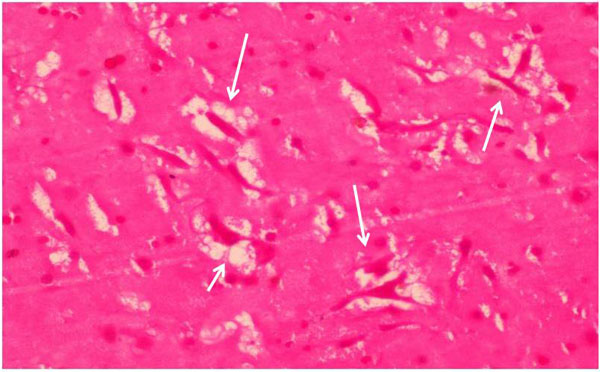
Histologic examinations showed pericardial invasion by a CM in 1 patient. During the operation, adhesions of pericardial sac was released from the myocardium. The CM extended into the inferior vena cava. There was a large solid tumour (length 13 cm.) (Fig. 4) Histologic examination demonstrated a polypoid lesion composed of stellate cells in a myxoid vascular stroma, with no atypia seen, in keeping with a benign atrial CM (Fig. 5) Typically, the pathological changes observed included proliferation of capillaries, blood extravasation, and disseminated fibrin deposits (Fig. 6). The average dimension of the resected CMs was 3.9 ± 1.1 cm (median, 4.9 cm; range: 2.8-13.0 cm).
3. RESULTS
No hospital mortality was seen. Mean ICU and mean length of hospital stay was 9.6±2.4 days (5-19 days). Among the 35 long-term survivors, 14 patients were in NYHA Class-I, 10 patients were in Class-II, 9 patients were NYHA Class-III and 5 patients were NYHA-Class IV. No further peripheral artery embolic event or complication of central nervous system was observed, except in 1 patient. The main complications were pleural effusion, renal replacement therapy (n=2), LCOS (n=3), AF (n=5), pericardial effusion (n=1) and pneumonia (n=1). All incidentally verified CMs were solid tumours. Supraventricular or ventricular arrhythmias and valvular symptoms were more common in patients with solid CMs. During surgery in a patient with mitral valvular stenosis related to a large tumour, mitral valvular impairment was successfully repaired using an annuloplasty ring. Postoperative TEE showed no evidence of valvular insufficiency.
All patients underwent follow-up. During follow-up, 12 patients were in NYHA Class-I whereas 26 patients (68.4%) were in NYHA Class-II. Antiplatelet (aspirin or clopidogrel) agents and coumadin were prescribed in patients who underwent valve replacement or had AF. One cerebral thromboembolic event was observed in a 63 year old female patient 22 months after surgery. Recurrence of CM was detected 37 months after surgery in 2 patients who had a large CM. The recurrence was detected in the borderline of adjacent tissue of the posterior mitral valve and free wall of left atrium. This may be due to local embolization of sessile cardiac tumour occurring during the first procedure. We detected incidentally a small size of CM (1.6-2.1 cm) in the right ventricle 46 months after the first operation in a male patient without any cardiovascular symptoms. We successfully excised the CM and the patient was discharged in a good clinical condition.
Three patients who underwent resection of CMs died during the follow-up period. One patient (64 year old female) died because of heart failure 32 months after surgery. Another patient had lung cancer. The third patient who underwent coronary artery bypass grafting concomitantly with CM resection died because of myocardial infarction 49 months after surgery. For this series, the overall actuarial survival was estimated using the Kaplan-Meier method. Overall, survival for patients after surgical excision was 96.4±1.6% at 1 year, 91.7±2.4% at 5 years, 87.6±2.6% at 10 years and 85±1.9% at 15 years.
Clinical features of the sessile and papillary CMs were similar. CHF was more common in the left sided tumours which causes obstructive symptoms. Systemic or cerebral embolic events were more frequent in the papillary group. In agreement with previous publications, septal implantation was more common in the solid CMs, while papillary CMs were more likely to be extra-septal.
3.1. Pathologic Results
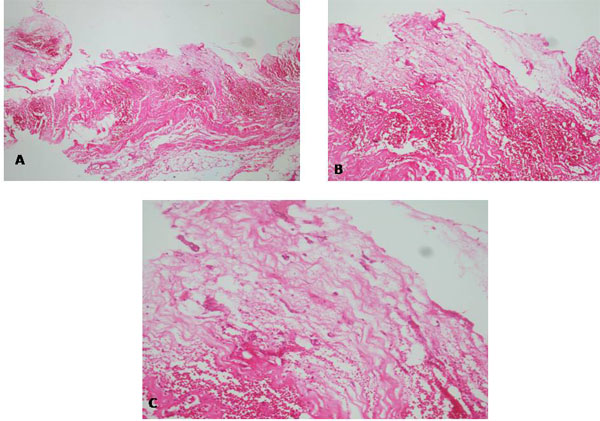
Mean tumour dimension was 2.1±0.4 cm (median: 2.3 cm) (range: 1.6-13 cm). Histologic examinations including embolic material showed that cardiac tumours contained a myxoid stroma with isolated round and spindle cells. Multinucleated cells were occasionally seen. The surface of cells were polygonal and in a monolayer. Macrophages, lymphocytes, and plasma cells were noted. Haemorrhagic areas were commonly found, in both CM types. Microscopic investigation of pericardium in a patient with nephrotic syndrome and pericarditis showed an invasion of pericardium by a myxoma included scattered spindle cells with scant pink cytoplasm and intrapericardial hemorrhgia (Fig. 7).
4. DISCUSSION
Malignant or beningn primary cardiac tumors are rare. The prevalence of cardiac tumors ranges from 0.0013% to 0.3% [4-11]. Myxoma is the commonest bening cardiac tumor (50% of all benign cardiac tumors). The aetiology of CMs is not clearly understood. The tumours may originate from primitive mesenchymal stem cells which have a capacity to differentiate along endothelial lines [9]. However, some markers suggest that intracardiac sensory nerve tissue can be the origin of CMs [10-12].
Our experience of CMs patients including age, gender ratio, type of CMs (papillar or solid), tumour location and clinical presentations were similar when compared with previous reports [4-12]. The majority of our patients were adults, except for 2 paediatric patients. Unlike previous publications, our series identified that proteinuria or renal failure may rarely be a first clinical presentation in these patients. Previous studies demonstrated that CMs may be associated with some urinary abnormalities [13] like membranous nephropathy [13], renal amyloidosis [14], interstitial nephritis [15] and cryoglobulinaemia [16]. The authors hypothesised that an antiendothelial cell antibodies might cause endothelial cell activation, apoptosis and cytotoxicity in the cardio-renal system. It is presumed that CMs can cause local activation of the immune system which allows the production of autoantibodies. Indeed, animal models showed the development of antiendothelial cell antibodies might cause severe proteinuria resulting from renal injury [17, 18].
To our knowledge we present for the first time a patient with CM with NHYA class IV combined with a pericardial invasion by a CM which was confirmed using histologic examination. Therefore, if a patient has severe pericardial adhesion concomitant with a large CM, a pericardial biopsy should be performed for histologic evaluation of pericardial invasion.
Ventricular tachyarrhythmia or peripheral or cerebral artery embolic events can be the primary symptom. Of the 38 patients retrospectively reviewed in our series, 13.1% had arterial embolic events [16-25]. Dyspnoea presented as the predominant obstructive cardiac manifestations in our series. In rare patients, some of the mediastinal masses can be misdiagnosed as CM because of cardiac external compression [26]. Magnetic resonance or computed tomography may provided the more detailed definition compared with TTE together with their extension to mediastinal structures [26-29]. TTE and TEE have a high sensitivity with detection rates reported to be nearly 100% [27-29]. In patients with CM, we suggest intraoperative TEE to identify intracardiac tumour metastasis and assess the results of valvuloplasty procedures during surgery.
Median sternotomy or right anterior thoracotomy provides an adequate mitral or tricuspid valve exposure for cases involving small or large CMs [30-34].
Recently, minimal invasive surgery with the guidance of a thoracoscope has been described for the treatment of intracardiac tumours [35, 36]. We used a right anterior thoracotomy for resection of CMs in some patients without any complication. In our opinion, our series will support the minimal invasive technique. Our experiences showed that annuloplasty or valve replacement with the use of minimal invasive approach may be performed safely. In this technique, we used the right anterior minithoracotomy incision using with femoral artery and central venous cannulation. We operated the patients with the use of normothermic or mild hypothermic cardiopulmonary circulation. If we needed bicaval cannulation, we used special designed central venous cannula underwent TEE.
In agreement with previous publications, a higher risk of perioperative complications would be expected in patients with NHYA Class-III and IV [37-39]. Because familial forms of CM have been reported, we recommend that the patients’ family have long-term echocardiography follow-up [40-44]. Lin et al. demonstrated that solid CMs were associated with more arrhythmias [45].
CONCLUSION
CMs can present with a wide range of symptoms or even be asymptomatic. Among our patients, those with a large solid CMs localized in the left atrium were associated with a greater incidence of CHF and obstructive symptoms. Proteinuria or acute renal failure may be the first sign of a right atrial CM. Peripheral or cerebral artery embolic events are related to tumour type and location. After diagnosis, early tumour excision should be performed. Surgery has excellent overall survival and freedom from reoperation. Follow-up using TTE is recommended.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by an Institutional Ethics Committee.
HUMAN AND ANIMAL RIGHTS
No Animals were used in this research. All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
We would like to thank to Prof. Omer Faruk Dogan for his supporting the preparation of this study, review prior to submission of re-revised manuscript, and description of different surgical method used in some patients.


