All published articles of this journal are available on ScienceDirect.
An Unusual Case of Fibromuscular Dysplasia with Bilateral Renal Macroaneurysms: Three-year Outcome After Endovascular Treatment
Abstract
Fibromuscular dysplasia (FMD) is an idiopathic, segmental, non-inflammatory and non-atherosclerotic disease that affects arterial walls, leading to stenosis of small and medium-sized arteries. FMD mostly involves renal and intracranial arteries and only in few patients is associated with macroaneurysms (RAAs). We present the case of a 45-years old woman with recent history of grade 2 hypertension that suffered of subarachnoid haemorrhage due to rupture of a basilar artery aneurysm. The cerebral aneurysm was immediately treated by coil embolization and an abdominal angio-CT scan was performed to investigate the presence of renovascular hypertension. The exam showed the presence of FMD of the renal arteries associated with presence of bilateral RAAs. Due to the high risk of rupture, the bigger aneurysm (2,5 cm diameter) present on the left artery was immediately treated by coil embolization. The fusiform aneurysm, present on the right renal artery, was instead treated one year later by using two flow diverter stents. After three years, an angiographic study showed that both cerebral and renal aneurysms were excluded from the blood flow without evidence of arterial restenosis.
INTRODUCTION
Fibromuscular dysplasia is an idiopathic, segmental, non-inflammatory and non-atherosclerotic disease of unknown origin, which affects the arterial walls, leading to stenosis of small and medium-sized arteries [1, 2]. FMD mostly involves renal arteries (58%) and intracranial arteries (32%) [3]. The clinical presentation may vary from an asymptomatic condition to a multisystemic disease depending on the arterial segment involved, the degree of stenosis, and the type of fibromuscular dysplasia [4]. Renal FMD mostly occurs in women between 15 and 50 years of age. In most cases, the patients have been asymptomatic for many years and this pathology is incidentally discovered. Less than 10% of renovascular hypertension is caused by FMD [4]. Three major types of FMD have been described: medial dysplasia, intimal fibroplasia and adventitial (periarterial) fibroplasia [4]. Medial fibroplasia, is the most common form accounting for 80–90% of all types of FMD and it is characterized by the typical “string of beads” appearance. The latter is due to multiple thin webs causing arterial stenosis with poststenotic dilatation [1, 2, 4]. Usually the “beads” appear like small aneurysms on angiography, but a few patients can develop renal macroaneurysms (RAAs) that have a propensity to rupture if not treated [5]. We report the case of a patient affected by FMD with renal bilateral RAAs that was successfully treated by using a combined endovascular approach.
PATIENT HISTORY
We present a case report of a 45-years old woman with a recent discovery of grade 2 hypertension. One month after the initiation of blood pressure treatment (amlodipine 10 mg once daily) the patient was admitted to the emergency room because of worsening headache and sudden appearance of vomiting. On admission, the blood pressure was 220/120 mmHg and a cerebral CT scan demonstrated the presence of subarachnoid haemorrhage. A cerebral angiography was performed showing the presence of a ruptured aneurysm of the basilar artery. The aneurysm was immediately treated through endovascular coil embolization. The patients recovered without neurological impairment but blood pressure remained difficult to control. To rule out the presence of renovascular hypertension, an angio-CT scan of the abdomen was performed and the exam highlighted the presence of bilateral FMD of the renal arteries with multiple RAAs. In particular, we documented the presence of a 2,5 cm diameter type 1 aneurysm in the left artery and a 1,3 cm diameter type 2 aneurysm in the right renal artery (Fig. 1). Due to the high risk of rupture an endovascular treatment of the aneurysm on the left side was immediately performed following a sequential approach that includes an initial, partial coiling of the defect followed by position of two stents and then conclusion of the coiling procedure. At the end of the treatment, the renal perfusion was optimal and the patient was discharged with improved blood pressure control although not yet optimal (150/90 mmHg during treatment with ace-inibitor, beta-blocker, thiazide diuretic and calcium channel blocker). A 3-month treatment with double anti-platelets therapy was prescribed (Aspirin 100 mg once a daily plus Clopidogrel 75 mg once daily). Radiological follow-up after two months showed full exclusion of both basilar and left renal artery aneurysm. Ten months later, due to persistence of resistant hypertension, we scheduled the treatment of the aneurysm on the right side. In this case the reconstruction of the right renal artery was performed by using two bridged stents Multilayer Cardiatidis. This approach allows obtaining a complete exclusion of the aneurysm from the renal flow maintaining a normal renal perfusion. The patient was discharged with prescription of three months of double anti-platelets therapy (Aspirin 100 mg once a daily plus Clopidogrel 75 mg once daily), followed by Aspirin alone. Three months after the second procedure an angio-CT scan showed normal renal vascularisation and exclusion of all treated aneurysms. During the follow-up visits we documented an improved blood pressure control (the last ambulatory blood pressure monitoring showed 24 hours blood pressure values of 114/64 mmHg in treatment with Atenolol 50 mg once daily plus Amlodipine 5 mg once daily) and the patient remained healthy, asymptomatic, with normal renal function. Three years after the first treatment, we performed a follow-up angiographic study that showed full exclusion of both cerebral and RAAs without any evidence of arterial restenosis (Fig. 2).
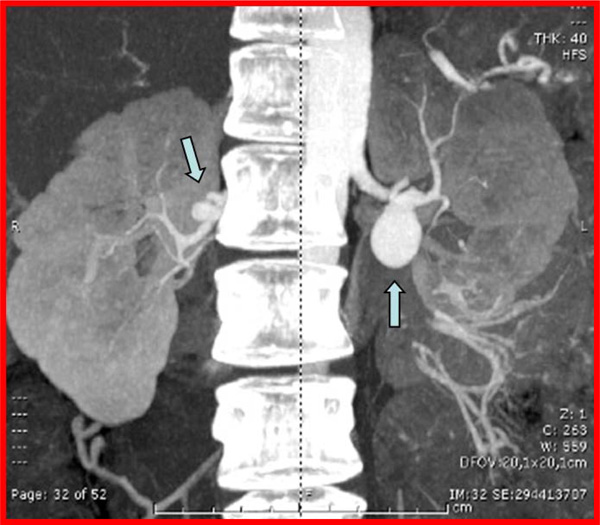
Abdominal angio-CT scan showing the presence of renal artery FMD with RAAs. The arrows indicate the presence of a type 1 aneurysm (2,5 cm diameter) in the left artery and a type 2 aneurysm (1,3 cm diameter) in the right renal artery.
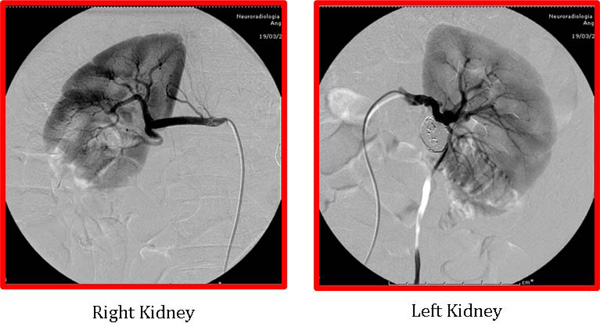
Angiographic images of the renal arteries after endovascular treatment. The saccular type 1 aneurysm on the left renal artery was treated by coil embolization. The fusiform type 2 aneurysm, present on the right renal artery, was treated by using two flow diverter stents. Three years after the first treatment the angiographic study showed that both renal aneurysms were excluded from the blood flow without evidence of arterial restenosis.
DISCUSSION
As mentioned above, renovascular FMD tends to affect mainly women and is the cause of almost 10% of renovascular hypertension [4]. Renal arteries are involved by FMD unilaterally in about 61% of cases, and bilaterally in almost 39%. At variance with atherosclerosis, FMD characteristically involves the middle and distal portion of the main renal artery. Gold standard treatment for FMD is percutaneous transluminal renal angioplasty (PTRA) because it is less invasive than surgical revascularization [2]. Moreover it can be performed on an outpatient basis, it is associated with lower morbidity and it is less costly. Although vascular stenting is widely used in atheroscleortic renal stenosis its use for FMD lesions treatment has been reserved as a “bailout” procedure in cases where suboptimal results are obtained with balloon angioplasty, or when complications such as acute occlusion or flow-limiting dissection are present [1, 4-6]. Nowadays, surgical revascularization is limited to patients who failed or are unsuitable for PTRA [7]. In the patient we presented, the FMD of the renal arteries was complicated by the presence of bilateral RAAs. True aneurysms may occur secondary to extreme post-stenotic dilatation or result from mural aneurysm that enlarge beyond perimuscular wall [7]. In any case the reported incidence of RAAs is low (0.015% –1.32%) and their association with FMD is an extremely rare event.
Circumstances necessitating repair of RAAs have been extensively debated. It is widely accepted that asymptomatic RAAs should be treated when their size is bigger than 2–2,5 cm and/or there is evidence of significant increase during serial radiological investigations. Smaller RAAs (< 2 cm) should be treated if associated with renovascular hypertension, high risk of dissection, significant arterial stenosis, intrarenal thrombo-emboli or renal infarctions. Finally, women with RAAs of childbearing age should be treated because of an increased risk of rupture during pregnancy (80%) [8, 9].
In the case presented here we first decided to treat the left renal aneurysm because of its size (2,5 cm) and the high risk of rupture due to the presence of renovascular resistant hypertension. On the other hand, the immediate treatment of the right renal aneurysm (1,3 cm) was controversial and we decided to follow-up the malformation time-course with serial radiological investigation. However, although the exams showed no changes in the aneurysm size, the blood pressure remained poorly controlled by the medical therapy. For this reason, we decided to treat the malformation.
Traditionally, RAAs have been treated surgically by using nephrectomy or variable reconstructive strategies such as aneurysm resection, aortorenal bypass, renorenal interposition, reimplantation and patch angioplasty [10]. These procedures are technically challenging, have a not negligible mortality, morbidity rates of 0-28% and imply prolonged recovery periods. Other potential risks include unplanned nephrectomy, branch occlusion, ureteral stricture, postoperative graft occlusion and postsurgical arterial stenosis [11, 12]. For these reasons, during the past decade different endovascular procedures have been proposed as an alternative to surgery. The various percutaneous approaches, similar to those developed for intracranial aneurysm treatment, are based on different embolization techniques or stent grafts, depending on malformation’s anatomy. The basic principle of these treatments is to exclude the aneurysms from the circulation either through packing with coils or by using covered stents as mechanical barriers [11].
Which endovascular procedure should be preferred to treat RAAs is still controversal. According to Runback et al. RAAs can be classified into three main types which have peculiar treatment strategies [9]. Type 1 RAAs include saccular aneurysms arising from either the main renal artery trunk or proximally from a large segmental branch. The relationship between the size of the aneurysm and the diameter of its neck is the most important factor driving the selection of the treatment for both cerebral and renal malformations, a treatment that is mainly based on coils embolization [12, 13]. Fusiform RAAs (type 2) cannot be treated with coils embolisation but they are eligible for covered-stent, a treatment that can be combined with PTRA in case of arterial stenosis. After covered-stent placement, the RAA is excluded without the risk of endotension, which is a potential cause of rupture [14, 15].
In our patient, both renal arteries and aneurysms had different anatomy. The left renal artery was rectilinear and the aneurysm was saccular (type 1) and for this reason it was treated with coils and stent. The right renal artery, instead, was twisting with the typical “string of beads” anatomy, and the presence of a fusiform aneurysm (type 2). Hence, this second aneurysm was treated by using two Multilayer Cardiatidis flow diverter stents obtaining a complete exclusion of the aneurysm from renal perfusion and midbranch artery flow preservation. The Multilayer stent is designed to decrease the mean velocity and vorticity within the aneurysm sac, thus favouring immediate thrombus formation and exclusion of the aneurysm from the circulation [11]. As far as we know, there is only one report of endovascular treatment of FMD associated with bilateral RAAs; in this case renal malformations were treated by using percutaneous stent-graft placement [15]. Moreover, there are only few reports about the use of stent plus coil embolization for the treatment of unilateral RAAs associated with FMD [14, 16-18].
CONCLUSIONS
The association between FMD, RAAs and hypertension is an extremely rare condition whose optimal treatment strategy is uncertain. In our patient with FMD and RAAs the renal arteries showed different morphology and type of aneurysm. For this reason two different endovascular approaches have been used and both of them resulted effective after a 3-years follow-up.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
ACKNOWLEDGEMENT
None declared.


