All published articles of this journal are available on ScienceDirect.
Anaemia and Long Term Mortality in Heart Failure Patients: A Retrospective Study
Abstract
Background:
Anaemia has been demonstrated as a risk factor in patients with heart failure over periods of a few years, but long term data are not available. We examined the long-term risk of anaemia in heart failure patients during 15 years of follow-up.
Methods:
We evaluated survival data for 1518 patients with heart failure randomized into the Danish Investigations of Arrhythmia and Mortality on Dofetilide (DIAMOND) trial. The follow-up time was from 13 to 15 years. After 15 years 11.5% of the patients were still alive.
Results:
Anaemia was present in 34% of the patients. 264 (17%) had mild, 152 (10%) had moderate and 98 (7%) had severe anaemia. Hazard ratio of death for patients with mild anaemia compared with patients with no anaemia was 1.27 (1.11-1.45, p<0.001), for moderate anaemia 1.48 (1.24-1.77, p<0.001) and for severe anaemia 1.82 (1.47-2.24, p<0.001), respectively. In multivariable analyses anaemia was still associated with increased mortality with hazard ratios of 1.19 (1.04–1.37, p=0.014) for mild anaemia, 1.23 (1.03–1.48, p=0.024) for moderate anaemia and 1.33 (1.07–1.66, p=0.010) for severe anaemia, respectively. In landmark analysis the increased mortality for mild anaemia was only significant during the first 2 years, while moderate anaemia remained significant for at least 5 years. There were too few patients left with severe anaemia after 5 years to evaluate the importance on mortality beyond this time.
Conclusion:
Anaemia at the time of diagnosis of heart failure is an independent factor for mortality during the following years but loses its influence on mortality over time.
INTRODUCTION
Anaemia is common in patients with heart failure (HF) and is found in 4%-55% of the patients [1-4]. Many studies have demonstrated anaemia to be a risk factor in patients with heart failure [5, 6] and anaemia is currently targeted in several interventional trials.
The majority of the studies evaluating the importance of anaemia are only reporting follow up for 2-3 years and just one study has a 5 year follow up [2]. To fully understand the implications of anaemia and the potentials of correcting anaemia it is important to know the risk associated with anaemia and HF over an extended period of time.
In the present study we therefore report the long-term effect of anaemia over 13 to 15 years of follow up. We furthermore explore differences in mortality rates between subgroups of anaemic patients in order to identify patients with a particularly high risk. We also perform landmark analysis to investigate whether the presence of anaemia remains significant during follow up.
MATERIALS AND METHODOLOGY
Patient Population
The current study is based on analysis of survival data including 1518 patients that entered into the Danish Investigations of Arrhythmia and Mortality on Dofetilide in Congestive Heart Failure—the DIAMOND-CHF trial. The design of the study has been described previously [7]. The screening population included 5,548 patients, of which 57 patients were excluded because of misrecorded personal data. Four patients were excluded from our study due to missing records of haemoglobin level, leaving 1514 for analysis. The DIAMOND trial was a multicenter, randomized, double-blinded, placebo-controlled study of the efficacy of a novel class III antiarrhythmic agent, dofetilide, in patients with acute myocardial infarction (MI) or HF. 34 Danish hospitals participated in the study. All patients admitted consecutively to their centres with new or worsening HF in the period between November 1993 and December 1995 were screened for entry in the trial. The centres represent approximately half the hospitals in Denmark that receive HF patients. Smaller county hospitals as well as university teaching hospitals participated, consequently making the study population highly representative of hospitalized HF patients in Denmark.
Inclusion criterias for the DIAMOND-CHF study were as follows: Patients should have been in New York Heart Association (NYHA) functional class III or IV at some time within the preceding month. The initial screening consisted of a clinical history, a physical examination, and an echocardiogram, which was recorded on videotape locally and evaluated in a central laboratory. Wall motion index (WMI), was measured using a 16-segment model of the left ventricle, and then calculated using a reverse scoring system (8). WMI multiplied by 30 gives a rough estimate of percent left ventricular ejection fraction and was obtained in 95% of the patients. Patients were eligible for the study if they had a WMI of no more than 1.2 (corresponding roughly to an ejection fraction of no more than 35 percent). Patients were excluded if they have had an myocardial infarction (MI) within seven days before screening, If they had a heart rate of less than 50 beats per min during waking hours, sinoatrial block or second- or third-degree atrioventricular block that was not treated with a pacemaker, a history of drug-induced proarrhythmia, a corrected QT interval exceeding 460 msec (500 msec in patients with bundle-branch block), a diastolic blood pressure of more than 115 mm Hg, a systolic blood pressure of less than 80 mm Hg, a serum potassium level of less than 3.6 mmol/L or more than 5.5 mmol per liter, recent use of class I or III antiarrhythmic drugs, a calculated creatinine clearance rate of less than 20 ml per min [9], serious liver dysfunction, acute myocarditis, planned cardiac surgery or angioplasty, aortic stenosis, cardiac surgery within the preceding four weeks, and the presence of an implantable cardioverter–defibrillator. There was no difference in mortality in the two groups of patients randomized to dofetilide or placebo.
Survival status was obtained from the Danish Central Personal Registry in 2008, 15 years after screening of the first patient. The follow-up time was 13 to 15 years. Follow-up was complete.
DEFINITION OF ANAEMIA
Anaemia was defined by the cut-off values defined by the World Health Organisation (WHO): haemoglobin (hgb) level lower than 120 g/L (corresponding to 7.5 mmol/L) in women and 130 g/L (corresponding to 8.0 mmol/L) in men denoted as WHO-anaemia throughout the manuscript. In order to examine if the prognostic importance of anaemia was driven by the subgroup of patients with most severe anaemia, the cut-off level of hgb was decreased with 10 and 20 g/L, respectively, resulting in three subgroups of anaemic patients: Mild, moderate and severe anaemia. Mild anaemia, corresponding for the first subgroup in each gender, was defined as hgb. lower than 120 g/L (6.8 mmol/L) in women, and lower than 130 g/L in men. Moderate anaemia was defined as hgb. lower than 110 g/L (6.8 mmol/L) in women, and lower than 120 g/L in men. And severe anaemia was defined as hgb < 100 g/L (6.2 mmol/L) in women and < 110 g/L in men.
STATISTICAL ANALYSES
Continuous variables are presented as medians with 5th and 95th percentiles, and discrete variables as percentages. Baseline characteristics were compared using the continuity-adjusted Chi-square test for discrete variables and analysis of continuous variables. Kaplan Meier estimators were used to construct mortality curves. Multivariable analysis was performed with Cox proportional hazard models. Hazard ratios were obtained from Cox proportional-hazards regression using all variables listed in Table 1. Model assumptions (proportional hazard, linearity of continuous variables and no interaction) were tested and found valid unless otherwise reported. Interaction analysis was made in Cox proportional-hazard models with inclusion of the interaction variable. A p value <0.05 was considered significant. All calculations were made using SAS software (SAS Institute, Cary, NC, USA).
Baseline Characteristics
| No Anaemia (n=1000) | Mild Anaemia (n=264) | Moderate Anaemia (n=152) | Severe Anaemia (n=98) | p-value | |
|---|---|---|---|---|---|
| Age | 68.4 (0.32) | 72.3 (0.58) | 72.7 (0.69) | 73.2 (0.89) | <0.001 |
| Weight | 77.2 (0.48) | 71.7 (0.92) | 69.2 (1.09) | 69.4 (1.25) | <0.001 |
| WMI | 0.87 (0.01) | 0.85 (0.02) | 0.87 (0.02) | 0.90 (0.02) | 0.33 |
| Creatinine clearance | 61.6 (0.79) | 50.0 (1.29) | 46.2 (1.35) | 43.6 (1.72) | <0.001 |
| Sex, male | 804 (81%) | 157 (60%) | 84 (55%) | 65 (66%) | <0.001 |
| Smoker | 365 (37%) | 73 (28%) | 47 (31%) | 33 (34%) | 0.04 |
| History of: | |||||
| Prior AMI | 507 (51%) | 135 (51%) | 81 (53%) | 54 (55%) | 0.82 |
| AF | 210 (21%) | 39 (15%) | 31 (20%) | 16 (16%) | 0.12 |
| Hypertension | 157 (15%) | 36 (14%) | 19 (13%) | 10 (10%) | 0.36 |
| NYHA III | 518 (52%) | 147 (56%) | 80 (53%) | 59 (61%) | 0.25 |
| NYHA IV | 51 (5%) | 21 (8%) | 20 (13%) | 7 (7%) | 0.003 |
| Betablocker | 96 (10%) | 30 (11%) | 16 (11%) | 10 (10%) | 0.85 |
| ACE-inhibitor | 767 (77%) | 184 (70%) | 103 (68%) | 73 (75%) | 0.02 |
| Diuretics | 951 (95%) | 257 (97%) | 159 (98%) | 92 (94%) | 0.16 |
Data are presented as mean, standard deviation (SD), number of patients (n) or percentages. WMI =Wall Motion Index. AMI = Acute Myocardial Infarction. AF = Atrial Fibrillation. NYHA = The New York Heart Association Functional Classification. ACE = Angiotensin Converting Enzyme.
Hazard Ratio for Death
| Mild Anaemia | Moderate Anaemia | Severe Anaemia | |
|---|---|---|---|
| 0-2 years | 1.34 (1.11-1.61, p = 0.003) | 1.22 (0.95-1.56, p = 0.115) | 1.44 (1.07-1.92, p = 0.015) |
| 2-5 years | 1.13 (0.88-1.46, p = 0.34) | 1.46 (1.06-2.01, p = 0.021) | 1.10 (0.71-1.69, p = 0.67) |
| 5-10 years | 0.97 (0.72-1.30, p = 0.82) | 0.93 (0.58-1.50, p = 0.76) | 1.48 (0.91-2.42, p = 0.11) |
Hazard ratio and 95% confidence interval.
ETHICS
The investigation conforms with the principles outlined in the Declaration of Helsinki II and was approved by the Central Danish Ethics Committee. All patients gave their written, informed consent before enrolment.
RESULTS
Patient Characteristics
According to the WHO criteria, 1000 (66%) patients had no anaemia, 264 (17%) had mild, 152 (10%) had moderate and 98 (6%) had severe anaemia. Baseline characteristics are shown in Table 1. Anaemia was associated with decreasing creatinin clearance, male gender, being smoker and in NYHA class IV.
MORTALITY ANALYSIS
Among the patients with no anaemia, 860 (86%) patients died during follow up, compared with 239 (91%) patients with mild anaemia, 143 (94%) patients with moderate anaemia and 97 (99%) patients with severe anaemia.
When evaluated in a univariate model, the hazard ratio of death for patients with mild anaemia compared with patients with no anaemia was 1.27 (1.11-1.45, p<0.001) for moderate anaemia 1.48 (1.24-1.77, p<0.001) and for severe anaemia 1.82 (1.47-2.24, p<0.001), respectively. To investigate whether the increased mortality in patients with anaemia reflected a higher prevalence of concomitant risk factors, a multivariable analysis, including age, gender, smoking, history of ischemic heart disease, previous MI, arterial hypertension, atrial fibrillation, NYHA functional class, WMI and creatinine clearance as covariates, was performed. Anaemia was still found to be a significant prognostic predictor of mortality with a hazard ratio of 1.19 (1.04–1.37, p=0.014) for mild anaemia, 1.23 (1.03–1.48, p=0.024) for moderate anaemia and 1.33 (1.07–1.66, p=0.010) for severe anaemia, respectively (Figs. 1 and 2).
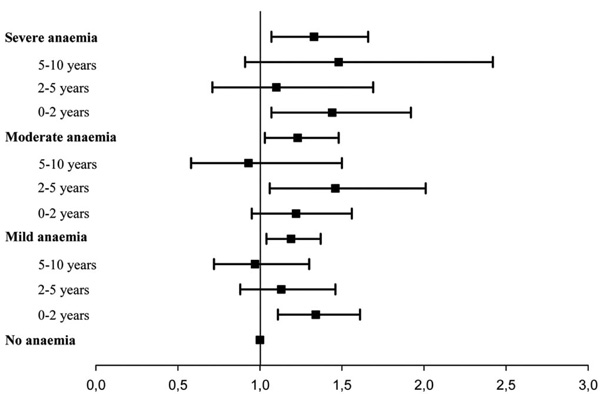
Hazard Ratio all cause death. Hazard ratios for death associated with anaemia in patients with chronic heart failure. Cox proportional hazards regression analysis adjusted for age, gender, smoking, history of ischemic heart disease, previous MI, arterial hypertension, atrial fibrillation, NYHA functional class, WMI and creatinine clearance. Bars indicate 95% confidence intervals.
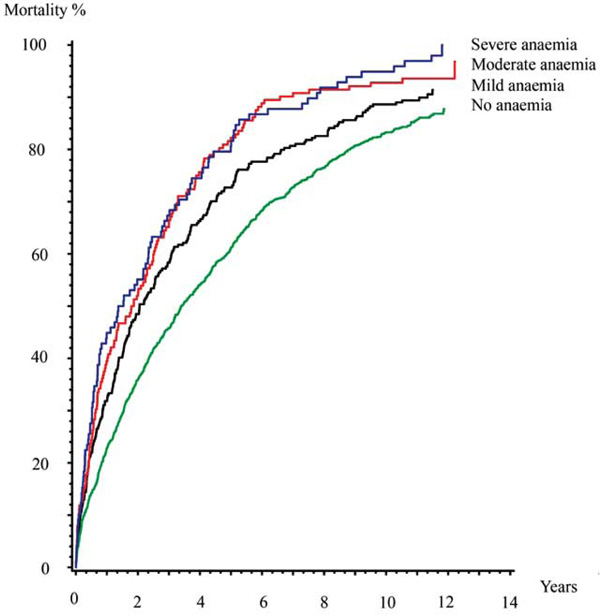
Mortality rates rates full follow up. Kaplain-Meier cumulative hazard estimates.
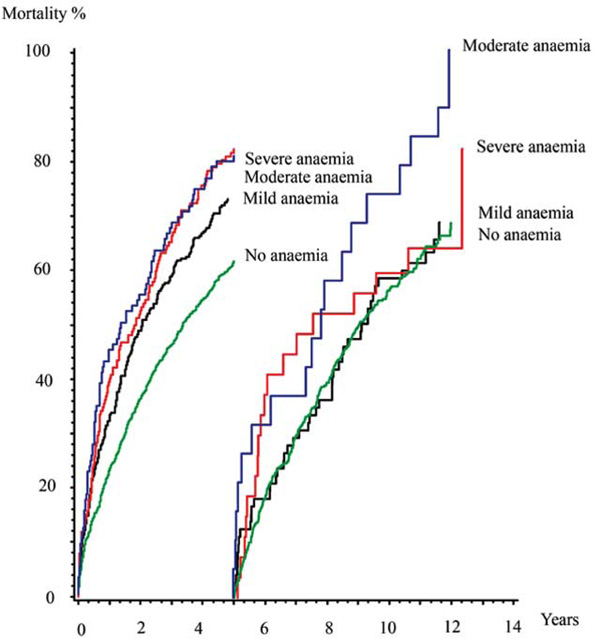
Mortality rates in land mark analysis. Kaplain-Meier cummulative hazard estimates.
Suspecting that anaemia loses its influence on mortality over time we performed a multivariable proportional hazard regression model investigating the mortality for predefined periods of time after the diagnosis. It confirmed that the increased mortality for mild and severe anaemia only was significant in the first two years with a hazard ratio of 1.34 (1.11-1.62, p=0.003) and 1.44 (1.07-1.93, p=0.015) respectively and the increased mortality for moderate anaemia was significant in the first five years, hazard ratio 1.46 (1.06-2.02, p=0.021) (Table 2, Fig. 3).
DISCUSSION
MORTALITY
This is, to our knowledge, the first long term study beyond 5 years of the prognostic importance of anaemia in patients with chronic heart failure. Other reports have described the prevalence and prognostic importance of anaemia in patients HF. However, these studies were based on relatively short periods of observation. In a recent meta-analysis with a follow up from 6 months to 5 years [2], anaemia was found to be an independent predictor of mortality HR 1.46 (1.26-1.69) p<0.001).
The present study extends previous reports by investigating the increased mortality risk related to anaemia at the time of diagnosis of heart failure is present during the following 13 to 15 years. We conclude that anaemia is an independent risk factor for mortality in HF patients, when assessing the mortality risk by using multivariable analyses. Furthermore, when assessing the importance of the severity of anaemia, we found that the mortality risk increased with the severity of the anaemia.
While we had a follow up of 13 to 15 years mild anaemia only had a short term influence on mortality while moderate anaemia remained an independent factor of mortality for at least 5 years. In the group of patients with severe anaemia mortality was particularly high resulting in the fact that there were too few patients left after 5 years too significantly evaluate the importance on mortality beyond this time.
In this study there were no signs of cancer at the time of inclusion leading to the argument that anaemia in the included patients was caused by chronic disease e.g. kidney disease. This is illustrated in our study by the fact that the more severe the anaemia the lower the creatinine clearance.
PERSPECTIVES
It is of great importance to know the long-term mortality risk for anaemic HF patients over an extended period of time in order to properly assess the risk-benefit of potentially correcting anaemia.
Anaemia is a novel therapeutic target in the treatment of HF patients though it still remains controversial whether to treat anaemia in patients with heart failure. The focus is on treatment with intravenous iron treatment and erythropoiesis-stimulating proteins to increase haemoglobin levels. There are several promising randomized trials investigating treatment with erythropoietin or darbepoitin with or without intravenous iron therapy versus placebo [10-14]. In relation to these trials there has recently been raised concern about the possibility of increased risk of tromboembolic events when raising haemoglobin levels. Currently a larger phase III trial is evaluating treatment of anaemic patients with heart failure with darbepoetin [15, 16].
LIMITATIONS AND STRENGTHS
Some limitations should be acknowledged. First, this study is retrospective. Second, generalisation of these data to other racial and ethnic groups should be cautious due to the fact that the Danish population remains largely white Caucasian. Second, information on the cause of anaemia, including ferritin and transferring levels, was not available for analysis. Third, a recent study [17] with serial haemoglobin measurements showed that persistent anaemia has a larger effect on mortality than transient anaemia. In our study the level of haemoglobin was not measured during follow-up, thus it remains unknown whether anaemia in the population was persistent or transient as well as there is no information about possible treatment of the anaemia.
The present study is strengthened by its very large sample size and by the fact that no patients were lost to follow-up.
CONCLUSION
The present study concludes that anaemia is present in one third of patients at the time of diagnosis of heart failure and that anaemia at the time of diagnosis of heart failure is an independent factor for mortality in the following 2-5 years. The presence of mild or severe anaemia loses its influence on mortality after 2 years while moderate anaemia loses its influence on mortality after 5 years.
COMPETING INTERESTS
The authors declare that they have no competing interests.


