All published articles of this journal are available on ScienceDirect.
Near Infrared Spectroscopy For Cerebral Hemodynamic Monitoring During Carotid Endarterectomy Under General Anesthesia
Abstract
Background:
Near infrared spectroscopy (NIRS) is a noninvasive method for continuous monitoring of cerebral oxygenation.
Objective:
To describe the intraoperative behavior of NIRS variables used to evaluate hemodynamic response in patients with atherosclerotic disease undergoing carotid endarterectomy under general anesthesia.
Methods:
Fifteen volunteers with atherosclerotic carotid disease with indications for endarterectomy were evaluated. After selection of patients, carotid stenosis was confirmed by appropriate diagnostic methods. NIRS was used for intraoperative monitoring. The variables total hemoglobin (Hb), oxygenated hemoglobin (O2Hb), deoxygenated hemoglobin (HHb), and regional oxygen saturation (rSO2) were evaluated at three intraoperative time points: before, during, and after carotid clamping.
Results and Discussion:
Measurements recorded by NIRS showed that, during the first 5 min of clamp time, patients experienced a decline in O2Hb levels, an increase in HHb levels, and a marked decrease in rSO2. Hb remained constant throughout the procedure. At the post-clamping time point, HHb, O2Hb, and rSO2 returned to patterns similar to those observed before clamping.
Conclusion:
NIRS was able to reliably and accurately identify the three stages of carotid endarterectomy and may predict the risk of cerebral hypoxia during carotid clamping under general anesthesia.
1. INTRODUCTION
Ischemic stroke is the most common cause of disability and the third leading cause of mortality in Western countries [1], and accounts for approximately 10% of all deaths worldwide. Nevertheless, its management remains a challenge [1, 2]. It is estimated that approximately one-fifth of all strokes in the adult population are caused by atherosclerotic disease of the carotid artery [1-4]. Knowing the natural history of this disease, preventing its development, securing an accurate diagnosis, prescribing the most appropriate course of treatment, and preventing sequelae and complications are essential to reducing these alarming statistics [5]. Ischemic cerebrovascular events are classified according to signs, symptoms, and findings on physical and neurological examinations, as well as on neuroimaging (computed tomography and magnetic resonance imaging). Based on clinical evaluation and timing of progression, they can represent transient ischemic attacks (TIAs) or cerebral infarcts, leading to varying degrees of disability [6-8]. In addition to stroke and TIA, the atherosclerotic disease has been associated in recent studies with changes in cognitive function and movement disorders [9, 10].
Treating carotid artery disease is a critical means of preventing stroke [11]. Several options are available for the management of atherosclerosis. Most are conservative, such as optimization of pharmacotherapy and changes in lifestyle habits [12, 13]. Surgical treatment of carotid stenosis can be performed by carotid endarterectomy or angioplasty and stenting [1]. Both seek to correct stenosis and prevent thromboembolism, but both induce complete cessation of cerebral blood flow through an artery already affected by stenosis [14-16].
To ensure safety in these procedures and minimize complication rates, intraoperative monitoring is essential. The methods applied must be reproducible, reliable, accurate, and noninvasive, and must be able to assess both hemodynamics and cerebral oxygenation if they are to prevent the complications of hypoxia [17-19].
Several methods have been used to monitor ischemia during surgical and endovascular procedures, such as neurocognitive assessment of the awake patient, measurement of reflux pressure in the carotid artery, somatosensory evoked potentials (SEPs), motor evoked potentials, electroencephalography (EEG), transcranial Doppler (TCD), and near-infrared spectroscopy (NIRS). However, there is no consensus as to which method is the gold standard, or even as to whether one is superior to others [2, 20-24].
NIRS is a method that uses the infrared region of the electromagnetic spectrum (600-900 nm) to measure O2 concentration. Near-infrared is the name given to the region immediately beyond the visible region in terms of wavelength, i.e., it is the region of the infrared spectrum closest to visible light. It was originally described by Jöbsis, and its use has grown in recent decades. It has several applications in clinical practice, including measurement of tissue oxygenation in muscles, connective tissue, and the lower limbs; more recently, articles have described its use in carotid artery surgery [25].
NIRS is a noninvasive method for monitoring cerebral autoregulation and oxygenation, and has most of the characteristics of an ideal monitor. This technology allows monitoring of oxygenated hemoglobin (O2Hb), deoxygenated hemoglobin (HHb), total hemoglobin (Hb), and regional cerebral oxygen saturation (rSO2), continuously and in real-time, from a small area of the frontal cortex. It thus provides an effective means of identifying individual limits facilitating patient management [26-31].
At a time when clinical outcomes are largely determined by the optimization of target organ functions, accurate perioperative monitoring is paramount [32, 33]. The information provided by NIRS can significantly change management during anesthesia [34]. The evidence shows that optimizing oxygenation of the brain and other vital organs reduces morbidity in various surgical procedures [35, 36]. Due to the advantage, it provides as a simple, continuous, noninvasive monitoring method, NIRS is a valuable tool for improving patient care in daily anesthesia practice [37-39].
1.1. Objective
To demonstrate the intraoperative behavior of the aforementioned variables (Hb, O2Hb, HHb, and rSO2) by NIRS in patients undergoing carotid endarterectomy under general anesthesia, specifically during the first 5 min after clamping the carotid artery, and compare this behavior to that observed during the pre-clamping and declamping periods.
2. MATERIALS AND METHODS
2.1. Patients and Methods
This study was approved by the Research Ethics Committee of the Universidade Estadual de Campinas (Unicamp), Campinas campus, with Certificate of Submission for Ethical Assessment (CAAE) number 09911113.2.0000.5404 and proof of submission number 021738/2013, both dated July 22, 2013.
In a cross-sectional, prospective, cohort-based design, 15 patients of both sexes, all aged over 50 years, with the carotid disease of atherosclerotic etiology, previously detected through clinical manifestations (TIA or stroke) and confirmed through Doppler ultrasonography of the carotid and vertebral arteries, were recruited from the outpatient Vascular Diseases clinic of Hospital de Clinicas da Unicamp. All patients had unilateral carotid stenosis ≥70% and a patent circle of Willis (right and left anterior cerebral arteries, anterior communicating artery, posterior cerebral arteries, right and left posterior communicating arteries) and vertebrobasilar system (right and left vertebral arteries and basilar artery), as confirmed by CT angiography of the cervical and cerebral vasculature. Patients who agreed to participate and provided written informed consent were included in the study.
After diagnostic confirmation by imaging, all patients underwent carotid endarterectomy with continuous intraoperative NIRS monitoring. After the procedure, the patients continued to receive follow-up at the outpatient clinic as per protocol [40].
Individuals with ongoing stroke and progressive symptoms were excluded from the sample, as were those with asymptomatic carotid stenosis detected on routine imaging; those who did not have a patent circle of Willis; those with neurodegenerative diseases; and those who, for any reason, refused to participate in any of the stages of the study.
To detect a difference of 10% in the parameters under study with a coefficient of variation of 30%, a two-sided 5% significance level, and a power of 80%, a sample size of 15 patients was necessary. The study would be terminated if a statistically significant number of patients were lost to follow-up, thus jeopardizing the research and its results.
A commercially available FD-DOS (diffuse optical spectroscopy) system (Imagent, ISS™ Inc., USA) was used for data acquisition. This device employs two optodes reflectively (i.e., both are on the same side of the surface) and continuous lighting as the excitation method. The system consists of a photomultiplier tube as the detector and four diode lasers as light sources, with a modulation frequency of 110 MHz. Each light source has different wavelengths, ranging from 690 to 840 nm. The optical probe was positioned over the prefrontal cortex ipsilateral to the stenotic carotid. The variables of interest (Hb, HHb, O2Hb, and rSO2) were recorded every 0.05 s. The collected NIRS data were entered into Microsoft Office® Excel 2016 spreadsheets for storage, statistical analysis, and preparation of graphs and tables.
These data were analyzed descriptively and used to plot graphs. To obtain a summary measure of the studied parameters, third-degree polynomials were fitted for each participant, and their coefficients were taken as representative of the behavior of the variables. Illustrative graphs were then plotted. This procedure allowed a comparison of the three stages of surgery. The Friedman test and Bonferroni correction were used for multiple comparisons [41-46]. A p-value <0.05 was considered significant.
3. RESULTS AND DISCUSSION
The study sample consisted of 15 patients (10 men and 5 women), who underwent carotid endarterectomy performed by the classic technique under general anesthesia. The mean patient age was 69.9 years (range, 56-79 years; standard deviation, 6.2 years). Most patients (n=14) were white; one self-identified as Brown. Considering the main comorbidities associated with atherosclerotic disease, 12 patients had hypertension, 10 had dyslipidemia, 8 had diabetes, and 13 were smokers. Regarding vascular conditions, arterial insufficiency in the lower limbs was present in three patients, two had abdominal aortic aneurysms, and three had a history of acute myocardial infarction. Two patients were obese (BMI >30 kg/m2). No patient had atheromatous involvement of the vertebral arteries, and all had an intact and patent circle of Willis.
Regarding the surgical procedure, the mean operative time was 102.5 min, ranging from 68 to 150 min, with a standard deviation of 28.2 min. All patients underwent clamping (mean duration, 21.1 min; range, 14 to 51 min; standard deviation, 8.7 min). Seven patients had surgery on the left carotid artery, and eight on the right.
Calcified and fatty atheromatous plaques were found during surgery, as confirmed by histopathological examination.
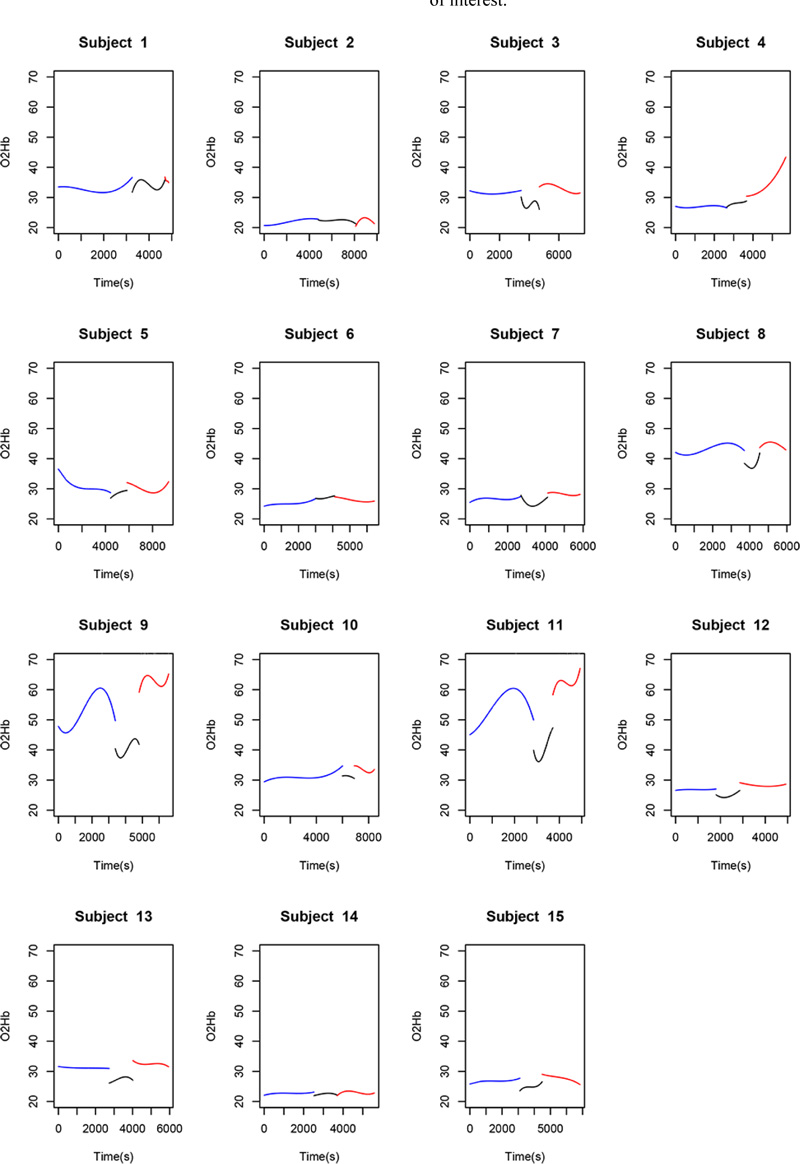
Observation of the behavior of the O2Hb variable during the course of the procedure revealed some points in common among certain patients. Of the 15 patients in the sample, 10 (66.7%) had a marked decline in O2Hb levels from the pre-clamping stage to the clamping stage and throughout this second stage, albeit to different degrees. During the declamping stage, the observed pattern returned to pre-clamping values, independently of the magnitude of the change.
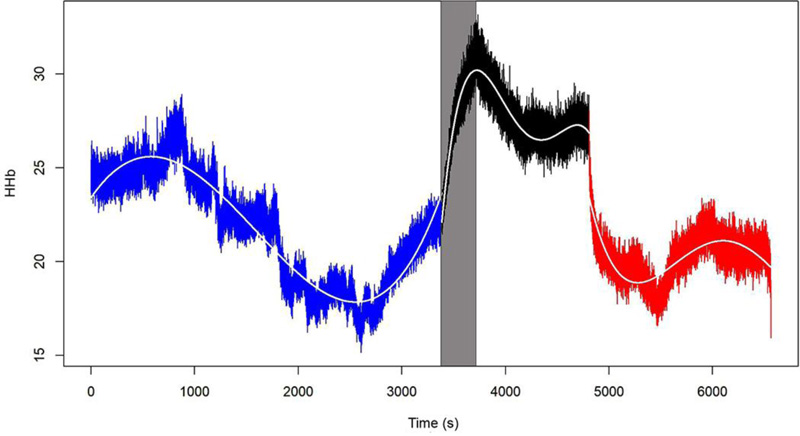
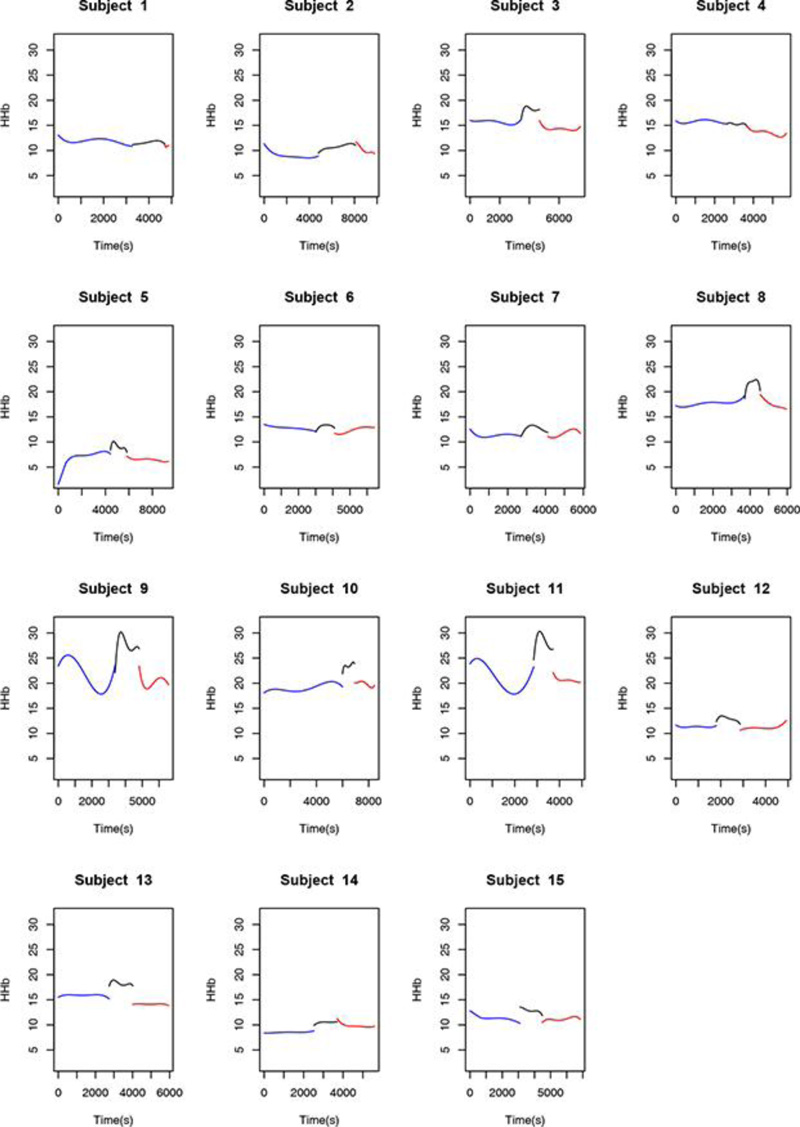
Observation of the behavior of the HHb variable during the course of the procedure also revealed points in common among certain patients. Of the 15 patients in the sample, 11 (77.3%) had a marked rise in HHb levels from the pre-clamping stage to the clamping stage and throughout this second stage, albeit to different degrees. During the declamping stage, the observed pattern returned to pre-clamping values, independently of the magnitude of the change.
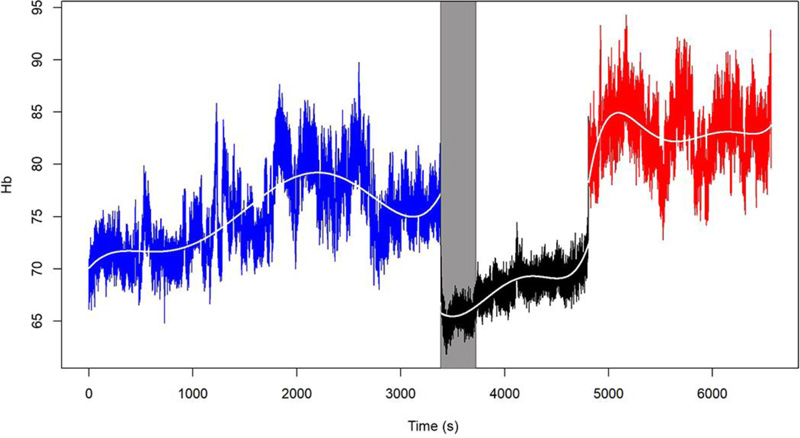
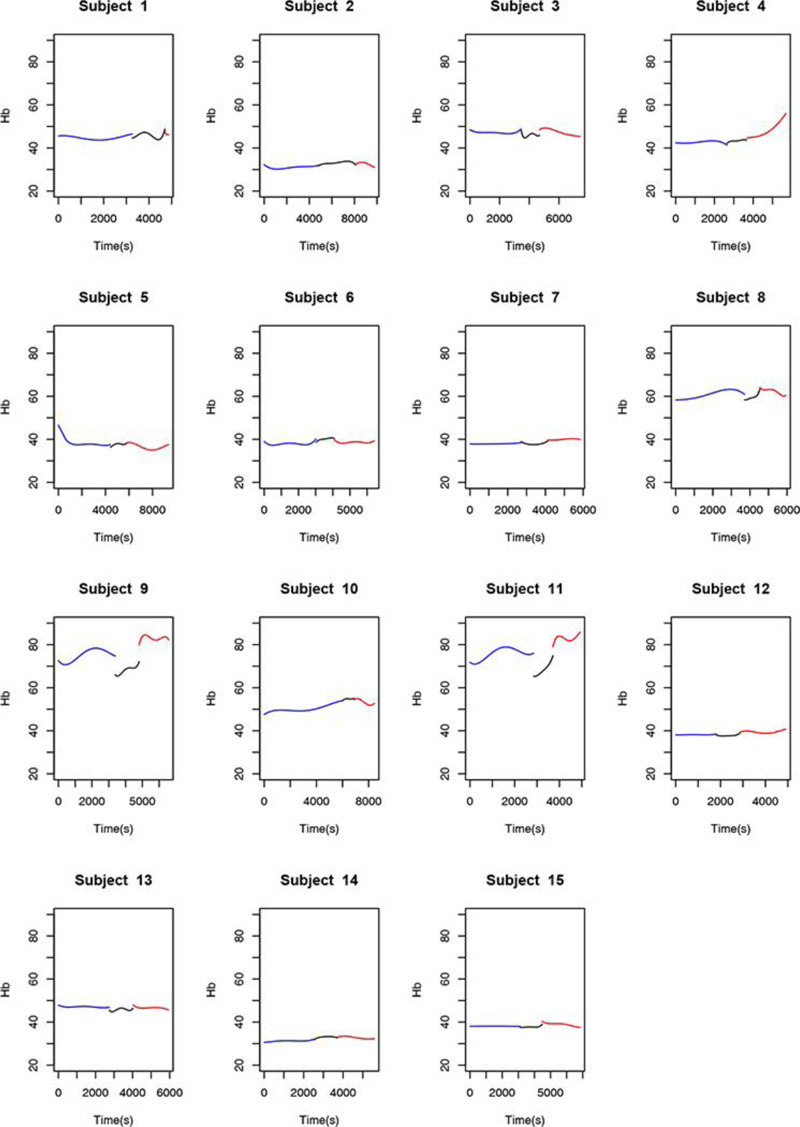
Observation of the behavior of the Hb variable throughout the procedure and taking into account the relationship between this variable, O2Hb, and HHb, there was no marked difference between stages in the study sample. Overall, for the Hb variable, there was less of a difference in data dispersion throughout the procedure.
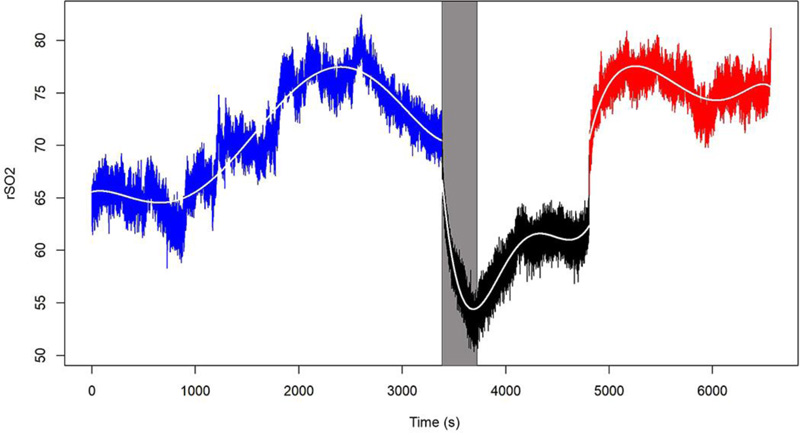
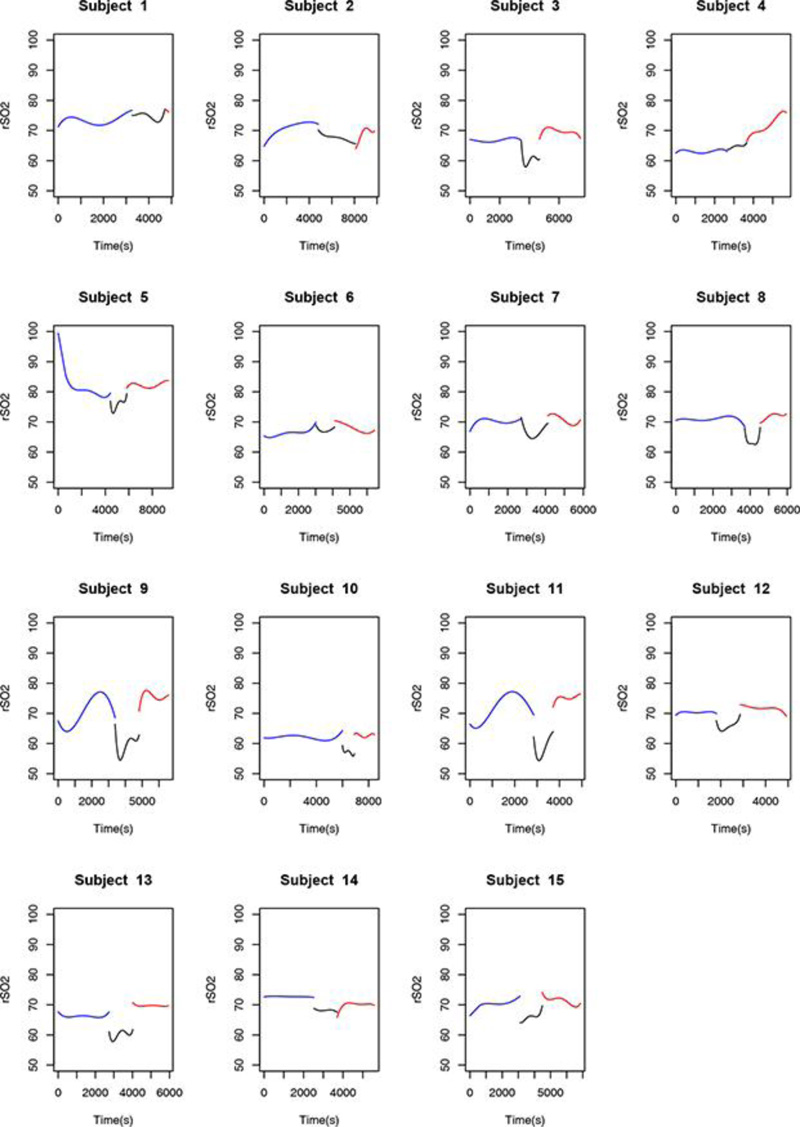
Finally, observation of the rSO2 variable throughout the procedure and considering its derivation from the variables O2Hb and Hb variables, a behavior common to most of the studied patients was detected. Of the 15 patients assessed, 12 (80%) had a marked decrease in rSO2 during the clamping stage of surgery, regardless of the magnitude of the data collected and of the dispersion between measurements.
The statistical analysis was divided into two parts. First, data from the NIRS spreadsheets were analyzed descriptively and used to plot graphs. To obtain a summary measure of the studied parameters, third-degree polynomials were fitted for each participant and their coefficients were taken as representative of the behavior of the variables. Illustrative graphs were then plotted.
- For the O2Hb variable, different behavior patterns were observed between the pre-clamping stage and the following stages.
- The behavior of HHb differed between the pre-clamping and unclamping stages.
- Finally, the rSO2 variable differed between the Pre-clamping stage and the 5-min clamp time point.
The behavior of the O2Hb, HHb, Hb, and rSO2 variables in one of the 15 patients is shown in Figs. (1-4), where the pre-clamping stage is in blue, the clamp time in black, the 5-min clamp time in shaded gray and the declamping stage in red. The variable in question is plotted on the vertical axis, and time in seconds on the horizontal axis. The colorless line, obtained through the fitted polynomials, represents the mean of the variable of interest.
In the second part of the statistical analysis, the summary measures obtained were described using descriptive measures, and the three stages of surgery (pre-clamping, 5-min clamp time, and declamping) were compared. In addition to the fitted polynomial coefficients, the mean value of the variables at each stage was considered to represent the behavior observed in patients over time. The Friedman test was used at this stage of the analysis.

- Mean O2Hb values differed between the pre-clamping stage and the subsequent time points. Descriptive analysis of O2Hb levels comparing the stages of the procedure showed significant differences between the pre-clamping and 5-min clamp time points, as well as between pre-clamping and declamping (p=0.001 for both comparisons). Comparison between the pre-clamping and 5-min clamp time points tended toward statistical significance (p=0.059).
- Mean HHb values differed across all three stages of the procedure. Descriptive analysis of HHb levels comparing the stages of the procedure showed significant differences between the pre-clamping and 5-min clamp time points, between the 5-min clamp and declamping stages, as well as between the pre-clamping and declamping stages (p=0.001 for all three comparisons).
- Mean Hb values differed between the pre-clamping stage and the subsequent time points. Descriptive analysis of Hb levels comparing the stages of the procedure showed significant differences between the 5-min clamp and declamping time points, as well as between the pre-clamping and declamping stages (p=0.001 for both comparisons). There was no significant difference between the pre-clamping and 5-min clamp time points (p=0.999).
- Finally, mean rSO2 values differed among all three stages of the procedure. Descriptive analysis of rSO2 levels comparing the stages of the procedure showed significant differences between the pre-clamping and 5-min clamp time points, between the 5-min clamp and declamping stages, as well as between the pre-clamping and declamping stages (p=0.001 for all three comparisons).
Fitted polynomials for each of the four variables (O2Hb, HHb, Hb, and rSO2) in each of the 15 patients are shown as mosaic plots (Figs. 5-8). The three stages of endarterectomy are represented as follows: pre-clamping in blue, clamping in black, and declamping in red. The horizontal axis shows operative time in seconds, and the vertical axis, is the variable of interest.
4. DISCUSSION
The prevalence of carotid stenosis increases with age; 5% of people over 65 years of age have >50% carotid stenosis, with the incidence being greater in men [13]. Patients with >50% carotid stenosis have a 1-5% risk of stroke [13]. Correlations exist between carotid stenosis, atherosclerotic coronary artery disease, and occlusive peripheral artery disease of the lower limbs [13]. Risk factors for atherosclerosis include age 65 years or older, high blood pressure, diabetes mellitus, dyslipidemia, and obesity. Some lifestyle habits, such as smoking and sedentary behavior, can facilitate disease progression; genetic predisposition may also be implicated [13].
Considering atherosclerotic carotid disease and its epidemiology, the findings of the present study are consistent with the literature. In our sample, the predominance of male patients, the greatest involvement of atherosclerotic disease between the fifth and seventh decades of life, and the overwhelmingly white racial makeup were all consistent with the literature [26]. Regarding risk factors for the development of the carotid disease of atherosclerotic etiology, we found that hypertension, dyslipidemia, and diabetes mellitus were major drivers of atherosclerotic disease in our patients and that smoking was an associated risk factor [26].
The first surgical reports on the treatment of cerebrovascular atherosclerosis were published in the early 1950s. Initial results were not promising due to suboptimal surgical technique. Over time, as the technique evolved, so did outcomes improve [7]. Enthusiasm for carotid endarterectomy was revived after publication of the results of the first randomized trials in 1990 [7]. With more recent data, carotid endarterectomy has emerged as an effective measure for stroke prevention in asymptomatic and symptomatic patients with severe carotid artery stenosis [7].
However, for carotid endarterectomy to be beneficial, the morbidity and mortality associated with the procedure must be minimal. One of the most serious complications of this procedure is the new onset or worsening of neurological deficit. The two leading causes of intraoperative cerebral ischemia are embolic events and decreased cerebral blood flow [8].
In our sample, there were no cerebral ischemic events and no deaths during hospitalization or in the first 6 months following the surgical procedures.
According to Rudzinski et al. [32], to maintain a sufficient cerebral blood flow to meet the metabolic demand imposed by neuronal electrical activity, the brain uses several mechanisms that aim to protect the central nervous system from fatal consequences arising from hypoxia and energy deficit; this is known as cerebral autoregulation [32, 38]. When these mechanisms are disrupted, life-threatening cerebral infarction can occur. Even if such ischemic brain injury is detected by magnetic resonance imaging, the diagnosis can be late and the damage, irreversible. Therefore, monitoring disorders of blood flow and autoregulation is extremely important in clinical practice, as decreased cerebral blood flow is completely silent until neuronal dysfunction approaches the level of permanent injury. Early recognition of decreased cerebral blood flow states can help identify and treat ischemic brain injury [47, 48].
Broadly, there are three different methods of brain monitoring: those that assess cerebral hemodynamics (TCD, carotid artery stump pressure or back-pressure); those that assess cerebral oxygen metabolism (NIRS and jugular monitoring); and those that assess brain state/function (electroencephalography and somatosensory evoked potentials). However, none of these can predict the occurrence of cerebral ischemia or prevent unnecessary shunting, especially not in procedures performed under general anesthesia [49-54].
Monitoring neurological status during locoregional anesthesia (wake-up test) seems to be the best method for predicting the need to perform a carotid shunt after clamping the internal carotid artery and can be considered the best method for intraoperative neurological evaluation [37, 55, 56].
At our facility, one of the eligibility criteria for performing carotid endarterectomy is adequate collateralization of the cerebral arterial circulation, i.e., adequate patency of the circle of Willis. In patients who do not meet these criteria, stent angioplasty of the carotid artery is performed instead. For both techniques, we routinely use cerebral monitoring; as we recently implemented NIRS for this purpose, this research reports our initial experience. All patients in the study had adequate collateralization of the cerebral arterial circulation, as verified by preoperative CT angiography, and thus underwent carotid endarterectomy by the classic technique. All procedures were performed under general anesthesia, which is the technique preferred by our department.
Currently, electroencephalography and TCD are the most widely used intraoperative monitoring methods to prevent cerebral ischemia and are considered the gold standard for cerebral monitoring during carotid endarterectomy. However, both have limitations [57]. Neither provides perfect sensitivity and specificity. Indeed, no single monitoring method achieves 100% sensitivity and 100% specificity compared with awake patient monitoring. According to the evidence, TCD, NIRS, and SEPs potentials for selective maneuvers provide similar sensitivity and specificity. SEPs provide the least precision. However, in clinical practice, it is often difficult to obtain a signal of sufficient quality with TCD monitoring, and it is also often difficult to avoid the movement of the transducer. Thus, TCD has not shown superior precision compared with SEPs or NIRS, both of which are easy to apply. Therefore, the use of these monitoring systems may prove superior in clinical practice [55, 58, 59].
EEG measures the electrical activity of the brain through electrodes placed on the scalp. However, it is time-consuming, difficult to interpret, and often influenced by some anesthetics. Furthermore, preexisting EEG abnormalities in patients with severe stroke can make EEG interpretation essentially impossible [32, 57].
TCD detects changes in cerebral blood flow by measuring velocities in the middle cerebral artery during and after carotid endarterectomy [57]. A 100% increase in postoperative cerebral blood flow is associated with a tenfold higher risk of cerebral hyperperfusion syndrome [57]. However, TCD cannot be performed in all patients, as the temporal bone window is absent in 10 and 15% of patients. In addition, the close proximity to the operating field carries a high risk of displacement of the Doppler transducer. In addition, TCD is an expensive and highly operator-dependent method. Especially for this subgroup, a reliable alternative monitoring technique is needed [57]. TCD is one of the methods being researched by our team and is the object of several ongoing studies.
Moritz et al. obtained better results with TCD, but there was no significant difference compared with stump pressure and NIRS. For TCD as well as for NIRS, the evidence shows that relative changes are more accurate for diagnosing cerebral ischemia than absolute values [55, 60, 61]. Thus, we considered relative values in the present study, taking baseline values for each of the patients in relation to the four variables monitored by the NIRS, following their progression over the three stages of endarterectomy, and comparing the behavior of these variables.
NIRS is suggested as an alternative for cerebral monitoring as it is non-invasive, user-friendly, and it allows instantaneous and continuous monitoring of cerebral O2Hb reduction produced by systemic hypoxemia. This method may be particularly useful in identifying a threshold for postoperative risk of cerebral hyperperfusion syndrome [57], as well as in head trauma and acute stroke. Although most intraoperative neurological sequelae occur due to embolism, NIRS is used mainly to detect hypoperfusion through increased cerebral oxygen extraction during carotid clamping [51, 62-69].
Most variables analyzed during anesthesia focus on oxygen supply but do not analyze the balance between oxygen supply and demand. At a time when clinical outcomes are largely determined by optimizing target organ functions, NIRS is a valuable tool for optimizing patient care in daily practice [37, 70]. NIRS may become the preferred intraoperative monitoring technique in the near future, even for patients under general anesthesia [32, 71-73].
In this study, we observed that 66.7% of the patients had a precipitous decline in O2Hb levels throughout the clamping stage as compared with the pre-clamping stage, with the resumption of pre-clamping patterns during the post-unclamping period. For the HHb variable, 77.3% of the volunteers showed a marked increase in levels during carotid clamping. During the post-clamping stage, the observed pattern also resumed pre-clamping behavior.
A recent study with TCD and NIRS demonstrated that patients with symptomatic carotid occlusion had lower velocity values measured by Doppler and a smaller increase in SaO2 detected by NIRS than asymptomatic patients. It also found that O2Hb can represent a marker of hemodynamic status in carotid disease and that NIRS can discriminate symptomatic from asymptomatic patients [74]. Thus, NIRS can make an important contribution to explaining the pathophysiological mechanisms of stroke occurrence [18, 59].
NIRS has disadvantages: only local conditions in the frontal lobe, which is irrigated by the anterior cerebral artery, are measured, and perfusion changes in other areas of the brain may escape detection [41, 57, 75, 76]. There is no consensus in the medical literature as to the best location for the placement of NIRS probes. Considering that this method is still undergoing validation for cerebral monitoring purposes and the anatomy of the blood supply provided by the anterior cerebral artery, we chose to place the probes over the frontal lobe in all patients.
Although extracranial interference by factors like movement and the cardiac cycle can influence readings [70], this risk can be reduced by focusing on changes in oxygenation, using a dual-detector system, and performing sequential clamping of the arteries [52]. The authors of this study believe that oscillations in the NIRS recordings may also be attributable to the cardiac cycle and gas exchange. Based on these recommendations and aiming to reduce potential errors, we took oxygen saturation as the main NIRS parameter and clamped the carotid arteries sequentially.
The main issue with using NIRS during carotid endarterectomy is that the oxygen saturation value below which neurological dysfunction occurs remains undefined [8]. Several studies have observed an abrupt mean decrease of 7.2% in SaO2 (compared with baseline) during carotid clamping. Regional cerebral oxygen saturation (rSO2) reached a new plateau within 5 min and remained significantly low after 15 min of clamping, followed by a return to baseline once the clamp was removed. There were no neurological deficits intraoperatively, during the cerebral deoxygenation period, or postoperatively [8].
In another study, NIRS was used for ipsilateral brain monitoring, in addition to TCD and the wake-up test. A percent drop in regional oxygen saturation and a decline in mean flow velocity on Doppler were detected after carotid clamping. A drop in rSO2 >20% or in mean flow velocity >50% was considered an indicator of cerebral ischemia that could predict the need for shunt insertion [77].
Stilo et al. [78] performed carotid endarterectomy under local anesthesia. Regional bilateral cerebrovascular oxygen saturation was monitored in all patients. Changes after carotid artery clamping were recorded. A drop >20% was considered an indicator of cerebral ischemia that could predict the need for carotid shunt, but actual shunting was only performed based on the wake-up test. Compared with pre-clamping values, a significant reduction in rSO2 was found in the cerebral hemisphere ipsilateral to the operated artery, while no significant change was observed contralaterally [24, 78, 79].
Wang et al. [80] used TCD and NIRS concomitantly to assess cerebral SaO2 and concluded that a decline of up to 12.3% in SaO2 during clamping (as compared with the pre-clamping baseline) is acceptable.
We are preparing a detailed analysis of the variations in regional oxygen saturation detected by the NIRS, in order to propose a future classification correlating the risk of adverse events with the decline in rSO2 values. In the present study, we found that, between the pre-clamping and clamping stages and throughout the carotid clamping stage, 80% of the volunteers experienced a marked decrease in rSO2, 66.7% experienced a precipitous decline in O2Hb levels, and 77.3% showed a significant increase in HHb levels. Hb remained constant throughout the procedure. At the post-clamping time point, HHb, O2Hb, and rSO2 returned to patterns similar to those observed before clamping. Thus, the ability of NIRS to detect variations in oxygenation instantly and differentiate hemoglobin and saturation behavior at the three stages of surgery is evident, even in this small sample. NIRS is a promising tool for the study of cerebral hemodynamics.
CONCLUSION
NIRS is capable of measuring changes in total hemoglobin, oxyhemoglobin, reduced hemoglobin, and oxygen saturation at the three stages of carotid endarterectomy under general anesthesia (pre-clamping, clamping, and unclamping), noninvasively and in real-time.
During the first 5 min of carotid clamping, the following phenomena occur:
- Maintenance of total hemoglobin levels
- Decline in oxyhemoglobin levels
- Increase in reduced hemoglobin levels, and
- A drop in oxygen saturation in relation to pre-clamping values.
Despite these changes, compensation occurred throughout the clamping period, with the gradual reestablishment of pre-clamping levels and no neurological sequelae in any of the patients evaluated.
LIST OF ABBREVIATIONS
| TIA | = Transient Ischemic Attack |
| CVA | = Cerebrovascular Accident |
| TCD | = Transcranial Doppler |
| EEG | = Electroencephalogram |
| FD-DOS | = Diffuse Optical Spectroscopy |
| O2Hb | = Oxygenated Hemoglobin |
| HHb | = Deoxygenated Hemoglobin |
| Hb | = Total Hemoglobin |
| rSO2 | = Regional Oxygen Saturation |
| SEP | = Somatosensory Evoked Potentials |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the Research Ethics Committee of the Universidade Estadual de Campinas (Unicamp), Campinas campus, Brazil, with Certificate of Submission for Ethical Assessment (CAAE) number 09911113.2.0000.5404 and proof of submission number 021738/2013, both dated July 22, 2013.
HUMAN AND ANIMAL RIGHTS
No animals were used that are the basis of this study. All human procedures were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Informed consent was obtained from all individual participants included in the study.
STANDARDS OF REPORTING
STROBE guidelines and methodologies have been followed.
AVAILABILITY OF DATA AND MATERIALS
All relevant data are within the paper.
FUNDING
The authors have no financial relationships relevant to this article to disclose.
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
ACKNOWLEDGEMENTS
Declared none.


