Impact of Magnesium L-Lactate on Occurrence of Ventricular Arrhythmias in Patients with Implantable Cardioverter Defibrillators: A Randomized, Placebo-Controlled Trial
Abstract
Background:
We evaluated the antiarrhythmic efficacy and quality of life (QoL) impact of oral magnesium Llactate on patients with an implantable cardioverter defibrillator (ICD).
Methods:
This prospective, double-blind, placebo-controlled trial randomized 70 patients with an ICD to receive oral magnesium L-lactate 3 tablets twice daily (504mg elemental magnesium daily) or matching placebo for 12 months. Patients were seen at baseline, 12, 24, 36, and 52 weeks. The primary endpoints were the cumulative occurrence of ICD therapy [either shock or anti-tachycardia pacing (ATP)] or QoL between the groups.
Results:
Among the 70 randomized patients with a mean ± SD follow-up of 6.4 ± 4.1 months, 10 patients in the placebo group and 8 in the magnesium group experienced either ICD shock or ATP [HR 0.84, 95% CI 0.33 to 2.12; p=0.706]. Without significant arrhythmia suppression, only minimal effects on QoL were seen. Eighty six percent of all patients had serum intracellular magnesium deficiency.
Conclusion:
In our underpowered trial, patients with ICDs had intracellular magnesium deficiency but oral magnesium Llactate only nonsignificantly reduced the occurrence of ICD therapies and had little impact on HrQoL.
INTRODUCTION
Implantable cardioverter defibrillators (ICDs) are used to prevent sudden cardiac death in patients at high risk or those with a past history of ventricular fibrillation [1-3]. The occurrence of 1 or more shocks has shown to be independently associated with reductions in mental well-being and physical functioning (p<0.002 vs. baseline for both) [4]. There are few studies suggesting antiarrhythmic drug use in ICD patients can reduce the number of ICD shocks, although many have undesirable adverse events [5]. In a pilot study of 20 patients with a history of arrhythmias, we previously found that 63.2% of patients had baseline intracellular magnesium concentrations below the normal reference range [6]. Supplementation with magnesium L-lactate (504 mg of elemental magnesium daily) for 48 hours increased intracellular magnesium concentrations significantly in that study (p=0.002) [6, 7]. If intracellular magnesium deficiency is a driver of arrhythmogenic events in patients with an ICD, proper oral magnesium replenishment may reduce risk of ICD shocks and improve health-related QoL (HrQoL). Thus, the object of this trial was to evaluate the impact of oral magnesium L-lactate on the cumulative occurrence of ICD shocks and patient-perceived HrQoL.
METHODS
This was a randomized, double-blind, placebo-controlled trial conducted at an urban teaching hospital (Hartford Hospital) in Hartford, Connecticut. It was approved by the Institutional Review Board with written informed consent [ClinicalTrials.gov (NCT00282620)]. Trial participants were men and women over 18 years of age with either a newly-implanted ICD or recent ICD shock (within the past 6 months). Patients were excluded if they had an inability to swallow, a noncardiac disease with a survival prognosis of less than 12 months, hypermagnesemia, creatinine clearance <30mL/min, lactic acidosis, or previous intolerance to magnesium L-lactate.
Seventy eligible patients were randomized to receive either magnesium L-lactate (Mag-Tab SR; Niche Pharmaceuticals) 3 tablets twice daily (504 mg of elemental magnesium per day) or placebo for 52 weeks. Patients were to be evaluated at baseline, 12, 24, and 52 weeks. Demographic data, including patient age, sex, ejection fraction, indication for ICD, and current medications were collected at baseline. ICD therapy occurrence (either defibrillation shock or anti-tachycardia pacing), HrQoL questionnaires, and serum magnesium concentrations were performed at each time-point. Intracellular electrolyte determinations (magnesium, calcium, sodium, potassium, and phosphate; EXAtest, Intracellular Diagnostics, Foster City, CA) were performed at baseline. In EXAtestTM, buccal cells are bombarded with high-energy electrons and emit x-rays with wavelengths that are distinct and unique to the elements and atoms within them.
HrQoL was measured using the self-administrated Ferrans & Powers QoL Index (QLI) Cardiac Version IV and the Short Form-36 Version 2.0 (SF-36) [8, 9]. The QLI scores range from 0 to 30, with 30 being superior QoL. The SF-36 is a multi-purpose, short-form health survey with 36 questions that produces two summary measures: a physical component summary (PCS) and a mental component summary (MCS) [10]. Each SF-36 scores range from 0 to 100 points, with higher scores indicating superior QoL.
The primary outcomes were the cumulative incidence of an ICD therapy and changes in HrQoL. Analyses were performed on all randomized patients reaching the 12 weeks assessment period. HrQoL scores are presented as mean values by treatment group and follow-up time. The primary and secondary outcomes were analyzed using the Student’s t-test or Mann-Whitney U test when appropriate. Kaplan-Meier survival analysis for ICD-therapy-free survival was compared by the log-rank test. Dichotomous variables are presented as percentages and were compared between groups using a χ2 or Fisher’s exact test where appropriate. Statistical analyses were performed with SPSS version 20.0 (SPSS Inc., Chicago, IL).
Given an annual ICD discharge reduction rate of 2.5 events per year with a standard deviation of 5.5 ICD therapy occurrences annually, the sample size per group would be 77 people assuming an alpha=0.05 and power=0.8 [11]. For HrQoL, a sample size per group of 89 people would be needed to determine a 10 point difference in score between groups with a power=0.80 with an alpha=0.05 [10-15].
RESULTS
We were unable to recruit the anticipated number of subjects and overall tolerability to experimental and placebo therapy was poor (Fig. 1). Of the 70 randomized patients, only 21 patients (9 magnesium L-lactate and 12 placebo) completed the 52-week follow-up. Baseline characteristics of 50 randomized patients who completed at least one follow-up appointment at 12-week were similar between magnesium L-lactate and placebo treatment groups (Table 1).
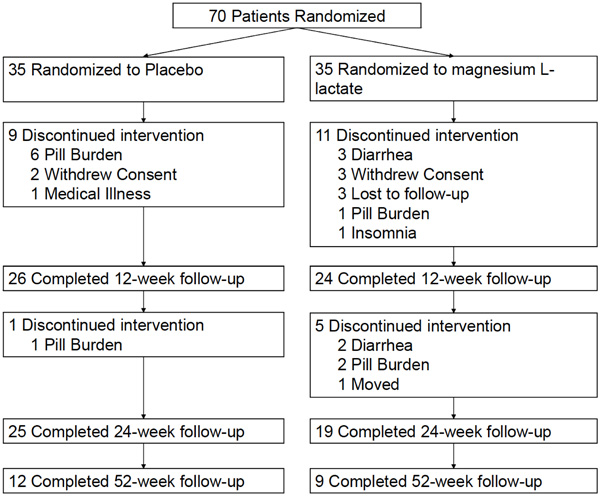
Trial profile.
Baseline characteristics of randomized patients that completed at least 1 follow-up appointment.
| Variable | Placebo (n=26) |
Magnesium L-lactate (n=24) |
|---|---|---|
| Age (years) | 68.0 ± 10.4 | 61.0 ± 13.1 |
| Gender (Male) (%) | 17 (65.4) | 21 (87.5) |
| Ethnic Origin (%) | ||
| White | 22 (84.5) | 18 (75.0) |
| Black | 2 (7.7) | 5 (20.8) |
| Hispanic | 2 (7.7) | 1 (4.2) |
| Primary Prevention (%) | 19 (73.1) | 19 (79.2) |
| Secondary Prevention (%) | 7 (26.9) | 5 (20.8) |
| History of Alcohol Use (%) | 13 (50.0) | 9 (37.5) |
| History of Cigarette Smoking (%) | 4 (16.7) | 9 (37.5) |
| History of Diabetes (%) | 6 (23.1) | 6 (25.0) |
| History of Hypertension (%) | 20 (76.9) | 17 (70.8) |
| History of Myocardial Infarction (%) | 19 (73.0) | 14 (58.3) |
| History of Heart Failure (%) | 15 (57.7) | 18 (75.0) |
| Baseline Serum Creatinine (mg/dL) | 1.08 ± 0.28 | 1.09 ± 0.24 |
| Baseline Serum Magnesium (mEq/L) | 2.23 ± 0.19 | 2.18 ± 0.19 |
| Baseline Intracellular Electrolytes (mEq/IU) | ||
| Magnesium | 32.5 ± 2.0 | 32.4 ± 1.7 |
| Calcium | 4.14 ± 0.9 | 4.34 ± 1.7 |
| Sodium | 4.53 ± 0.9 | 4.04 ± 0.9 |
| Potassium | 124.28 ± 33.4 | 127.0 ± 46.9 |
| Phosphorus | 16.14 ± 2.0 | 16.79 ± 2.8 |
| Baseline Medication Use (%) | ||
| Beta-Blocker | 23 (88.5) | 22 (91.7) |
| CCB | 2 (7.7) | 1 (4.2) |
| ACEI | 18 (69.2) | 13 (54.2) |
| ARB | 3 (11.5) | 7 (29.2) |
| Diuretics | 13 (50.0) | 13 (54.2) |
| Antiarrhythmics | 5 (19.2) | 2 (8.3) |
| Mean Systolic Blood Pressure (mm Hg) | 126.5 ± 19.3 | 123.8 ± 13.5 |
| Mean Diastolic Blood Pressure (mm Hg) | 75.1 ± 14.9 | 73.2 ± 7.4 |
Data are presented as mean ± SD, or n (%)
ACEI=Angiotensin converting enzyme inhibitor; ARB=angiotensin receptor blocker; CCB=calcium channel blocker
Mean QoL index (cardiac version) scores.
| Subscale | Baseline (n=68) | 12 Weeks (n=45) | 24 Weeks (n=30) |
|---|---|---|---|
| Total | |||
| Magnesium Placebo |
23.42 ± 3.37 24.68 ± 4.18 |
23.18 ± 4.11 24.10 ± 4.91 |
23.88 ± 3.77 23.92 ± 3.85 |
| Health & Functioning | |||
| Magnesium Placebo |
22.57 ± 3.79 23.57 ± 4.56 |
22.40 ± 5.26 22.91 ± 5.52 |
23.43 ± 4.82 22.41 ± 4.33 |
| Socioeconomic | |||
| Magnesium Placebo |
23.06 ± 4.94 24.91 ± 4.42 |
23.43 ± 3.57 24.64 ± 4.69 |
23.00 ± 3.56 24.09 ± 4.02 |
| Psychological/Spiritual | |||
| Magnesium Placebo |
23.86 ± 4.36 25.44 ± 5.98 |
23.70 ± 4.14 24.90 ± 5.23 |
23.93 ± 4.14 24.99 ± 4.40 |
| Family | |||
| Magnesium Placebo |
25.33 ± 4.48 26.78 ± 3.25 |
24.59 ± 4.57 25.88 ± 5.56 |
25.75 ± 4.60 26.60 ± 3.87 |
Mean SF-36 (norm-based) change from baseline scores.
| Subscale | Baseline (n=51) | Change from BL at 12 weeks Months (n=51) | Change from BL at 24 weeks (n=51) |
|---|---|---|---|
| Physical Function | |||
| Magnesium Placebo |
71.487 ± 7.744 69.273 ± 8.131 |
0.943 ± 4.658 1.052 ± 5.062 |
0.581 ± 5.788 0.191 ± 4.942 |
| Role Physical | |||
| Magnesium Placebo |
60.459 ± 11.448 58.397 ± 11.385 |
-4.307 ± 9.248 -0.668 ± 9.262 |
-3.716 ± 9.431 1.002 ± 9.550 |
| Bodily Pain | |||
| Magnesium Placebo |
51.740 ± 8.697 54.075 ± 12.274 |
-1.618 ± 6.620 -1.806 ± 11.830 |
-1.355 ± 8.685 0.096 ± 9.915 |
| Vitality | |||
| Magnesium Placebo |
50.062 ± 8.595 50.605 ± 8.192 |
-0.969 ± 4.578* 3.264 ± 7.856 |
-0.431 ± 4.917 2.696 ± 7.419 |
| General Health | |||
| Magnesium Placebo |
54.907 ± 8.889 57.683 ± 10.013 |
-1.101 ± 5.995 -1.213 ± 4.473 |
-1.118 ± 5.559 -0.325 ± 3.160 |
| Social Function | |||
| Magnesium Placebo |
55.563 ± 8.854 54.803 ± 13.069 |
-2.821 ± 6.932* 2.231 ± 6.870 |
-2.445 ± 7.373* 2.231 ± 6.660 |
| Role Emotional | |||
| Magnesium Placebo |
56.051 ± 12.806 58.696 ± 8.007 |
-3.220 ± 9.179 -0.880 ± 13.569 |
-1.743 ± 8.477 1.590 ± 11.610 |
| Mental Health | |||
| Magnesium Placebo |
54.071 ± 5.353* 58.696 ± 8.008 |
0.097 ± 4.355 -0.512 ± 8.182 |
0.000 ± 3.366 0.640 ± 6.202 |
| Physical Component Score | |||
| Magnesium Placebo |
37.540 ± 5.887 39.462 ± 5.296 |
1.311 ± 4.893 0.305 ± 6.073 |
1.427 ± 4.452 0.004 ± 5.469 |
| Mental Component Score | |||
| Magnesium Placebo |
50.364 ± 10.216 46.130 ± 13.069 |
1.847 ± 5.560 -0.771 ± 8.487 |
1.041 ± 6.377 -2.064 ± 7.029 |
* p<0.005 vs. placebo.
Eighty six percent of patients, regardless of randomization, had serum intracellular magnesium deficiency (intracellular concentrations below the reference range of 33.9–41.9 mEq/dL) but no patients had a serum magnesium concentration below the reference range of 1.6–2.7 mEq/dl at baseline (Table 1), 12 weeks (2.37±0.2 vs. 2.38±0.2; p=0.90), or 24 weeks (2.41±0.2 vs. 2.35±0.3; p=0.61). There were no significant differences between groups in intracellular electrolytes (including sodium, magnesium, potassium, phosphorous, or calcium) (Table 1).
Eight patients in the magnesium group and 10 patients in the placebo group experienced an ICD therapy [HR 0.84, 95%CI (0.33-2.12; p=0.706)]. An ICD shock occurred in 3 patients in the magnesium group and 4 patients in the placebo group (p=0.779) while anti-tachycardia pacing occurred in 7 magnesium patients and 8 placebo patients (p=0.870).
No significant differences were seen between groups in QLI scores at 24 weeks (p>0.27 for all scores, Table 2). For the SF-36 (Table 3), only two significant differences were found. Significant differences in change from baseline of Social Function scores were seen with magnesium L-lactate vs. placebo at 12 (2.23±6.87 vs. -2.82±6.93; p=0.013) and 24 weeks (2.23±6.66 vs. -2.45±7.37; p=0.022). Similarly, significant differences in the SF-36 MCS were also seen at 12 weeks (52.21±8.62 vs. 45.360±1.30, respectively; p=0.04), and 24 weeks (51.40±9.28 vs. 44.07±1.31; p=0.03).
DISCUSSION
Previous trials have found that antiarrhythmic drugs can reduce the number of ICD shocks but HrQoL was not assessed. A small randomized study concluded that the use of anti-tachycardia pacing to reduce the number of ICD shocks needed to terminate ventricular arrhythmias improves QoL [16]. We assumed that if we could suppress ICD shocks in our trial, that HrQoL would be similarly improved.
Intravenous magnesium has previously been shown to convert polymorphic ventricular tachycardia to normal sinus rhythm and to prevent atrial arrhythmias from occurring after cardiothoracic surgery [17, 18]. However, oral magnesium hydroxide 250mg twice daily failed to improve sinus rhythm maintenance at 1 and 6 months among patients with a history of atrial fibrillation in two previous studies [18, 19]. We felt the difference between the results of intravenous magnesium sulfate and oral magnesium hydroxide studies were related to the bioavailability between the two products (100% versus ~2%). We hoped that a formulation of magnesium with a higher bioavailability would produce superior antiarrhythmic results to oral magnesium hydroxide therapy.
In a small previous trial, we found that patients with arrhythmias had a normal serum magnesium concentration but a selective intracellular magnesium deficiency after buccal smear and analysis [6]. We were able to correct this deficiency in that trial after administering 504mg of magnesium L-lactate (a product with 41% bioavailability) daily for 48 hours [20]. EXAtest® buccal cell analysis is a reproducible intracellular ion test and magnesium concentrations correlate well (r=0.68, p<0.002) with myocardial intracellular magnesium concentrations [21]. We felt this intracellular magnesium deficiency was potentially clinically relevant because intracellular magnesium concentrations go down in mongrel dogs as they develop heart failure (36.9 to 33.9mEq/L, p<0.001); a prime risk factor in the development of ventricular arrhythmias [22].
In this current trial, we chose a 504mg of oral magnesium L-lactate dose to normalize the intracellular magnesium deficiency and hoped this would translate into a reduction in ICD therapy and an improvement in HrQoL. Since this was a natural therapy and ICD shocks are unpleasant and painful, we anticipated that patients would take magnesium therapy even though the number of tablets (3 large tablets twice daily) was extensive. This assumption was incorrect and resulted in slower than anticipated recruitment and numerous patient withdrawals. Patients simply did not want to add 6 additional tablets per day to the extensive number of drugs they were currently taking.
Interestingly, we found that 86% of ICD patients had baseline intracellular magnesium deficiency but no deficiency in other intracellular ions or a deficiency in serum magnesium. This is even greater than in our preliminary trial but may be expected since the severity of cardiovascular disease in this population was more extensive. Treating this deficiency with magnesium L-lactate led to a nonsignificant 16% reduction in ICD therapy but without recruiting enough patients and having many other patients truncating their therapy duration, there were not enough patients experiencing ICD therapy to truly evaluate the effectiveness of therapy. Since we did not reduce ICD therapy occurrence significantly, we would not have anticipated an impact of HrQoL either. Magnesium Lactate had minimal effect in changes in HrQoL compared with placebo over the 24 week study period in our trial.
We feel that we were overly aggressive in moving from our pilot trial into this large clinical trial. Had we known that patients would be unwilling to enroll in this trial and not tolerate this large number of tablets, we would have continued to research the impact of lower magnesium L-lactate doses on the intracellular magnesium concentrations in smaller preliminary trials. Perhaps after correcting the deficiency with 48 hours of intensive magnesium therapy, a smaller maintenance dose would be adequate to sustain normal intracellular magnesium concentrations thereafter. Once the minimal dose needed to maintain concentrations was known, we would have brought that into a large clinical trial. Moving forward, we will be evaluating these alternative regimens and still believe in the concept of correcting underlying physiologic mechanisms of arrhythmogenesis versus suppressing them with antiarrhythmic drug therapy.
CONCLUSION
Due to the extensive pill burden of our experimental regimen, recruitment was less than anticipated and drop-out rates were high. In our underpowered trial, a vast majority of patients with ICDs had intracellular magnesium deficiency but oral magnesium L-lactate only nonsignificantly reduced the occurrence of ICD therapies and had little impact on HrQoL. Before this therapy can be investigated further in ICD patients, additional preliminary studies assessing easier-to-tolerate regimens should be conducted.
FUNDING SOURCE
The study was supported by the Hartford Hospital Research Endowment, Hartford, CT and the Patrick and Catherine Weldon Donaghue Medical Research Foundation, West Hartford, CT. Niche Pharmaceuticals, Roanoke, TX provided drug and matching placebo, while Intracellular Diagnostics, Inc., Medford, OR provided intracellular electrolyte determinations.
CONFLICTS OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
This study was presented as a poster presentation at the American College of Cardiology 63rd Annual Scientific Sessions on March 29th, 2014 in Washington, DC.


