All published articles of this journal are available on ScienceDirect.
Is Vitamin D Deficiency a New Risk Factor for Cardiovascular Disease?
Abstract
The role of vitamin D in the regulation of bone metabolism has been well established. However, in recent years, many studies have demonstrated that its role extends far beyond bone health. Growing evidence has shown a strong association between vitamin D deficiency and hypertension, metabolic syndrome, diabetes mellitus and atherosclerosis. The mechanisms by which vitamin D exerts its cardiovascular protective effects are still not completely understood, but there is evidence that it participates in the regulation of renin-angiotensin system and the mechanisms of insulin sensitivity and activity of inflammatory cytokines, besides its direct cardiovascular actions. In this review, several studies linking vitamin D deficiency with cardiometabolic risk as well as small randomized trials that have evaluated the cardiovascular effects of its supplementation are presented. However, large randomized placebo-controlled studies are still needed before we can definitively establish the role of vitamin D supplementation in the prevention and control of cardiovascular disease.
1. INTRODUCTION
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality in the world [1]. Although the role of traditional risk factors is already consolidated, it is known that they cannot fully explain the development of CVD, which has caused continuous search for new risk factors. Growing evidence, obtained in recent years, has suggested that vitamin D deficiency may be associated with an increased risk of CVD [2].
Vitamin D is actually a steroid hormone whose primary function is the regulation of calcium and phosphorus homeostasis, through its interaction with parathyreoid gland, the kidneys and intestines. Although it can be obtained through food intake, the main source of vitamin D is represented by its synthesis in the body itself [2].
The role of vitamin D in regulating bone metabolism is well established for decades. In this function, it promotes increased intestinal absorption and renal reabsorption of calcium, acting also in its mobilization from the bone, in the presence of parathyroid hormone (PTH). Thus, diseases such as rickets and osteomalacia have been classically attributed to prolonged deficiency of vitamin D [2]. However, in recent years several studies have shown that the function of vitamin D in the body extends far beyond bone health, including the regulation of the immune system and anti-proliferative effects on cells, and may play an important role in the physiology of the cardiovascular system. Thus, vitamin D deficiency has been associated with disorders as varied as autoimmune diseases, infections, adverse maternal and fetal outcomes and various types of cancer, besides CVD [3]. However, whether vitamin D deficiency represents a new cardiovascular risk factor and also whether its oral supplementation can reduce the incidence of cardiovascular events is still under debate.
Thus, in this review, besides presenting the basic aspects of the metabolism and physiology of vitamin D in the body, we discuss the association between its deficiency and the occurrence of CVD, as well as the possible mechanisms involved in this association and the impact of its replacement on the prevention and control of CVD.
2. PHYSIOLOGY AND METABOLISM OF VITAMIN D
Under normal conditions only about 10% of the vitamin D needed by the body is achieved by ingestion of food, both in the form of vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol). Thus, the main source of vitamin D is represented by its synthesis in the body itself, which begins in the skin. When exposed to ultraviolet radiation, the skin vitamin D precursor 7-dehydrocholesterol undergoes a photochemical cleavage, yielding the previtamin D3. Such labile molecule, in a 48 hour period, undergoes a molecular rearrangement resulting in the formation of vitamin D3. Both the vitamin D coming from the diet, via intestinal absorption, and that formed from the previtamin D3 in the skin bind to circulating proteins and are transported to the liver where it is hydroxylated at the carbon 25, giving the 25-hydroxyvitamin D3 [25(OH)D]. This is then hydroxylated again, this time at carbon 1, at mitochondrial level in cells of the proximal convoluted tubules, as well as possibly in many other tissues, under the action of 1α-hydroxylase enzyme (1α-OHase), finally yielding the 1,25-dihydroxyvitamin D3 [1,25(OH)2D], its biologically active form (Fig. 1). This phase activation is tightly regulated by the serum levels of PTH, calcium and phosphorus. Although biologically inert, however, is the 25(OH)D, the circulating form in a larger quantity and more stable, which is the measured and adopted in clinical practice to assess the vitamin D in the body [2, 3]. PTH, by the other way, is a peptide secreted in response to low circulating levels of calcium and phosphorus which stimulates calcium reabsorption in the kidneys and its removal from the skeleton and increases renal production of 1,25(OH)2D, as mentioned above [2].

Schematic representation of the biosynthesis of vitamin D.
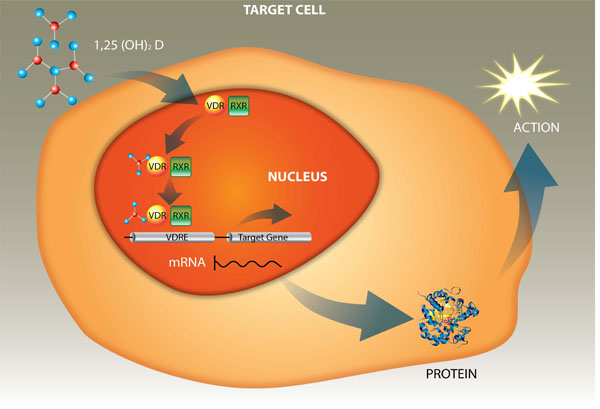
Schematic representation of the transcriptional control of gene expression by 1,25(OH)2D via VDR.
The complex mechanism of action of vitamin D inside the cells is already well established. Its active form crosses the cell membrane, enters the target cell and binds to a specific receptor, the VDR, which is present in the cytoplasm. This complex, 1,25(OH)2D-VDR, undergoes translocation to the nucleus and heterodimerizes with retinoic acid receptor (RXR). Next, the complex 1,25(OH)2D-VDR-RXR binds to the response elements of vitamin D (VDRE) in the deoxyribonucleic acid (DNA) increasing transcription to RNA of the genes responsible for the expression of proteins involved in the actions of vitamin D (Fig. 2) [4].
It is now known that the VDR type receptors are present in many cell types, including hematopoietic cells, lymphocytes, epidermal cells, pancreatic cells, myocytes, neurons, cardiomyocytes, vascular smooth muscle cells, endothelium and placental tissue, besides osteoblasts, osteoclasts, epithelial small intestine and renal tubular cells, which explains the multiplicity of non-calcemic actions taken by vitamin D for various tissues such as the endocrine, immune, central nervous and cardiovascular systems, as well as the numerous implications of their deficiency to health [3]. It is estimated that vitamin D controls, directly or indirectly, about 3% of the entire human genome. Thus, it is believed that a wide variety of physiological processes, including regulation of cytokines, inflammation, fibrogenesis, the renin-angiotensin system, immune response and cell growth and differentiation, may have direct or indirect influence of vitamin D [5].
3. PREVALENCE AND DETERMINANTS OF HYPOVITAMINOSIS D
The definition of vitamin D deficiency has also been a matter of debate. However, most agree that serum 25 (OH) D<2 0 ng/ml (or 50 nmol/l) is indicative of deficiency, in the range 20-30 ng/ml (or 50 to 75 nmol/l) match insufficiency and >30 ng/ml (or 75 nmol/l) represents vitamin D sufficiency [6].
According to studies conducted in several continents, vitamin D deficiency is one of the most common conditions in the world today. It is estimated that more than half of children and adults living in the United States, Canada, Mexico, Europe, Asia, New Zealand and Australia suffer from hypovitaminosis D [6].
Recent population survey in the U.S. showed that the prevalence of vitamin D insufficiency has doubled in the last 10 years, currently affecting not less than 90% of pigmented populations (blacks, Hispanics and Asians) and around ¾ of the caucasian population [7].
Several factors can interfere with the prevalence of hypovitaminosis D. Less sunlight exposure, that characterizes modern life in cities, coupled with the widespread use of sunscreen, stimulated by the fear of skin cancer, is undoubtedly the most important factor to the increasing prevalence of vitamin D deficiency in the world. In addition, in dark-skinned individuals cutaneous synthesis of vitamin D can be reduced by 50 to 90%, since melanin acts as a natural sunscreen. Additional factors include low latitude, winter, extremes of age, female gender, malnutrition, use of robes covering most of the body and obesity [6, 8].
4. ASSOCIATION BETWEEN VITAMIN DEFICIENCY AND MARKERS OF SUBCLINICAL ATHEROSCLEROSIS
Some studies, predominantly cross-sectional and small, have shown an association between serum 25(OH)D and markers of subclinical atherosclerosis and inflammation, although there remain some conflicting findings.
In a cross-sectional analysis of 119 type 2 diabetic patients of middle age, Bonakdaran and Varasteh [9] observed a significant association between deficiency of 25 (OH)D and both increased high sensitive C-reactive protein (hs-CRP) and the presence of microalbuminuria. However in a prospective observational study involving 227 type 1 diabetics, Joergensen et al. [10] reported that severe deficiency of 25(OH)D (<15.5 nmol/l) at baseline was not predictive of the development of microalbuminuria, although has been associated with higher total mortality.
Amer et al. [11], analyzing data from asymptomatic adults included in the National Health and Nutrition Examination Survey (NHANES) from 2001 to 2006, also found a significant inverse correlation between serum 25(OH)D and hs-CRP, independent of conventional risk factors, but only in those with levels of 25(OH)D ≤ 21 ng/ml, noting paradoxically a positive correlation between these two variables when they considered only the subgroup with levels of 25(OH)D > 21 ng/ml.
In a study involving 100 patients undergoing coronary angiography, a positive correlation between serum 25(OH)D and flow-mediated dilatation of the brachial artery (FMD) was observed, i.e., the lower the levels of 25(OH)D, the greater the degree of endotelial dysfunction [12]. Tarcin et al. [13], comparing 23 asymptomatic individuals with vitamin D deficiency (serum levels of 25(OH)D < 25 nmol/l) with a group with normal levels of the vitamin (average of 75 nmol/L) also found that the rate of FMD was significantly lower in the group with vitamin D deficiency.
In a cross-sectional analysis of a population-based cohort, the Rancho Bernardo Study, involving 654 healthy subjects, predominantly elderly (mean age 75.5 years), Reis et al. [2] observed, after multivariate analysis, an inverse correlation between serum levels of 25 (OH)D and intima-medial thickness (IMT) of the internal carotid artery. Significant inverse association, independent of conventional risk factors, between serum 25(OH)D and common carotid IMT was also observed in type 2 diabetics [14, 15].
In patients with chronic kidney disease, inverse correlation between serum 25(OH) D and measurement of carotid IMT was observed by Yadav et al. [16] in individuals at stages 4 and 5 not on dialysis, but not by Zang et al. [17], who included only patients with diabetic nephropathy.
And finally, Ross et al. [18], analyzing 149 HIV-positive patients, observed no association between serum 25(OH)D and inflammatory markers (tumor necrosis factor α, interleukin 6 and hs-CRP) nor endothelial markers (intercellular adhesion molecule-1 and vascular cell adhesion molecule-1).
5. CARDIOMETABOLIC AND VASCULAR IMPLICATIONS OF VITAMIN D DEFICIENCY
Results of several observational studies published in recent years have brought important evidence linking vitamin D deficiency with increased cardiovascular risk.
Evidence of the importance of vitamin D in the homeostasis of the cardiovascular system was initially suggested experimentally by the observation that knockout mice, lacking the VDR receptors, had impaired bone mineralization, small muscle fibers, suffered from high blood pressure (hypertension) and died of heart failure (HF) [5].
In the prospective cohort Third National Health and Nutrition Examination Survey (NHANES III), involving 3,408 elderly (≥ 65 years), followed for seven years, it was found, after multivariate analysis, that baseline levels of 25(OH)D were inversely associated with the risk of mortality from all causes, with an even stronger association for cardiovascular mortality [19].
In the Framingham Offspring study, involving 1,739 subjects with a mean age of 59 years, followed for five years, it was found that those with vitamin D deficiency [25 (OH)D<15 ng/ml] had a relative risk for the occurrence of cardiovascular events of 1.62 compared with those with levels ≥15 ng/ml, even after adjustment for conventional risk factors [20].
In the cohort Health Professionals Follow-up Study, which followed 18,225 male subjects, aged 40 to 75 years, for ten years, the authors found that participants with vitamin D deficiency [25 (OH) D ≤ 15 ng/ml] presented an incidence of myocardial infarction significantly higher than those with levels considered sufficient (≥ 30 ng/ml), with a relative risk of 2.42, after multivariate analysis [21]. A recent study involving 1,259 subjects showed that vitamin D status may also influence the prognosis of individuals already affected by myocardial infarction [22].
In the study Ludwigshafen Cardiovscular Health and Risk (LURIC), where 3,316 patients referred for a coronary angiogram were followed for seven years, it was evidenced that lower levels of both 25(OH)D and 1,25(OH)2D were independent predictors of fatal cerebrovascular accident (CVA) [23]. A similar result was obtained in the Copenhagen City Heart Study, a cohort that followed 10,170 individuals in a general population over 21 years, which also showed a clear independent inverse association between serum 25(OH)D and incidence of ischemic stroke [24].
Analyzing 90 elderly patients with stable HF and 31 controls, Ameri et al. [25] observed concentrations of 25(OH)D significantly lower in those with HF and found among them a prevalence of hypovitaminosis D (25 (OH)D<30 ng/ml) of almost 100%. They also observed that subjects with levels<10 ng/ml had left ventricle end-systolic and end-diastolic diameters and volumes greater and ejection fraction lower compared to those with levels ≥10 ng/ml. Low levels of 25(OH)D were also associated with greater prevalence of peripheral arterial disease [26] and abdominal aortic aneurysm [27].
The association between vitamin D deficiency and hypertension, metabolic syndrome (MS) and diabetes mellitus (DM) has also been investigated. Several analyses of the Third National Health and Nutrition Examination Survey (NHANES III) population, summarized in a review of Ullah et al. [28] have shown a clear inverse association between levels of 25(OH)D and blood pressure independently of numerous potentially confounding variables. In participants of two prospective cohorts, 613 men from the Health Professionals' Follow-Up Study and 1,198 women from the Nurses' Health Study followed for four years, the relative risk of developing hypertension in men with deficiency of 25 (OH)D (serum levels<15 ng/ml), after multivariate analysis, was 6.13 compared with those with levels ≥30 ng/ml. Among women, the same comparison revealed a relative risk of 2.67 [29].
In a population-based cross-sectional study carried out in two major Chinese cities, including more than 3,200 individuals, aged 50 to 70 years, it was found that lower levels of 25(OH)D were significantly associated not only with the presence of MS but with any of its individual components after multivariate analysis. The study also showed, in overweight and obese individuals, a significant inverse association between serum 25(OH)D and both fasting insulin and the insulin resistance index assessed by HOMA (homeostasis model assessment of insulin resistance) [30]. Association between vitamin D deficiency and MS, in smaller studies, was also observed in obese patients [31]and children [32].
In a cross-sectional analysis of 6,228 participants in the Third National Health and Nutrition Examination Survey (NHANES III), aged ≥20 years, the presence of DM was significantly associated with lower levels of 25(OH)D, after multivariate analysis, in non-Hispanic whites and individuals of Mexican origin, being observed, however, no such association among non-hispanic blacks [33].
Association between vitamin D deficiency and the occurrence of preeclampsia was also reported [34]. In a case-control study, in which women were followed from the first trimester until delivery, a basal concentration of 25(OH)D<15 ng/l was associated, independently, to a five times higher risk of occurrence of the disease [34].
Finally, in a meta-analysis published recently, which included 19 prospective studies and 65,994 individuals, Wang et al. [35] reported that the relative risk of the category of lower levels of 25(OH)D in relation to that of higher levels was 1.52 for all cardiovascular diseases, 1.42 for cardiovascular mortality, 1.38 for coronary artery disease (CAD) and 1.64 for stroke.
6. POSSIBLE MECHANISMS INVOLVED IN THE ASSOCIATION BETWEEN VITAMIN D DEFICIENCY AND CARDIOVASCULAR DISEASE
The mechanisms by which vitamin D exerts its cardio and vasculoprotective effects are not yet fully understood, being considered its regulatory effects on the renin-angiotensin system (RAS), glycemic control, inflammatory cytokines, the levels of PTH and calcium deposition in vascular smooth muscle, in addition to its direct vascular actions.
6.1. Vitamin D and Renin-Angiotensin System
It is well established that inappropriate stimulation of the RAS is associated with higher incidence of hypertension and CVD [28]. A good evidence favoring the role of vitamin D in the regulation of the RAS comes from experimental studies. In a study involving knockout mice, lacking the VDR, it was demonstrated an elevated production of renin and angiotensin II, causing hypertension, cardiac hypertrophy and increased water intake. These anomalies could be prevented by treating the mice with an angiotensin converting enzyme inhibitor or an angiotensin II receptor blocker. Based on these findings, it was concluded that vitamin D is a potent endocrine suppressor of renin biosynthesis [36]. On the other hand, in normal rats, it was demonstrated that vitamin D deficiency stimulates the expression of renin, while, if 1,25(OH)2D is administered, a reduction of renin synthesis occurs [36]. There is also evidence that PTH, whose serum levels may increase secondary to vitamin D deficiency, may also have a direct stimulatory effect on renin secretion [28]. Thus, the evidence strongly suggests that vitamin D deficiency may be implicated in the pathogenesis of hypertension via activation of the RAS.
6.2. Vitamin D and Insulin Sensitivity
The association between obesity, especially abdominal, and decreased insulin sensitivity is well established. On the other hand, it has been shown that obese patients have increased prevalence of vitamin D deficiency, which may be attributed to both a possible lower exposure to sunlight and its lower bioavailability, due to its greater sequestration by adipose tissue [28]. The demonstration of a strong independent inverse correlation between blood glucose and serum 25(OH)D [37, 38] coupled with epidemiological studies linking vitamin D deficiency with increased incidence of type 2 DM [33, 39] has suggested a major direct role of this vitamin deficiency in the pathogenesis of the disease. It seems that adequate levels of vitamin D in the body are essential in the process of insulin sensitivity, as indicated by some research. In the study by Chiu et al. [40], for example, involving 126 healthy adults, a clear positive correlation between serum concentrations of 25(OH)D and insulin sensitivity was demonstrated. On the other hand, there is also evidence that vitamin D deficiency is associated with impaired insulin secretion, via β cell dysfunction, as demonstrated in mice [41], which has been confirmed also by its association with the incidence of type 1 DM [42].
One mechanism that has been proposed to explain the association between vitamin D deficiency and obesity, hypertension, type 2 DM and other manifestations of MS involves the metabolism of calcium [43]. Low serum calcium levels, resulting from vitamin D deficiency, as we know, can lead to secondary elevation of PTH, which in turn promotes increased intracellular levels of this ion. The increase of intracellular calcium can lead to both increased differentiation of preadipocytes into adipocytes and inhibition of the function of GLUT4, an enzyme involved in cellular glucose uptake mediated by insulin [43].
6.3. Vascular and Cardiac Effects of Vitamin D
Experimental studies have demonstrated various protective actions of vitamin D directly on the heart and blood vessels. In cultured vascular smooth muscle cells from rabbit, Wakasugi et al. [44] demonstrated that the synthesis of PGI2, which plays an important role in reducing thrombogenicity, cell adhesion and proliferation of smooth muscle cells, increased significantly in the presence of 1,25(OH)2D. It is possible, therefore, that vitamin D acts as an important vasoactive agent and can thus exert a protective role against the development of atherosclerosis.
Moreover, it has shown its participation in regulating the expression of many proteins with vascular action, such as vascular endothelial growth factor, type 9 metalloproteinase, myosin, elastin, type 1 collagen and γ-carboxyglutamic acid, the latter a protein that protects the vessel against parietal calcification, and also in the suppression of pro-inflammatory cytokines, including interleukin-6 and tumor necrosis factor-α in vitro and in vivo [45].
There is also experimental evidence that its antiatherogenic action involves specific effects on the immune system, including a direct effect on naive CD4 T cells, inducing the development of T helper type 2 (Th2) lymphocytes, responsible for the production of interleukin-10 (IL-10), which inhibits macrophage activation, a key step in the process of atherogenesis [46]. Furthermore, inhibits the transcription of interferon-γ (IFN-γ), secreted by T helper type (Th1) cells, that is, on the contrary, a potent activator of macrophages and suppressor of Th2 lymphocytes [47].
In relation to the heart, there is evidence that vitamin D plays an important role in the modulation and maintenance of cell structure and function. The treatment with 1,25(OH)2D increases expression of myotrophin, a cardiac muscle protein, and decreases expression of atrial natriuretic peptide, which is inversely related to cardiac function. Furthermore, the treatment with 1,25(OH)2D increases the expression and nuclear localization of the VDR in cardiac cells [48]. Thus, the suppression of the effects of vitamin D fully justifies the development of myocardial hypertrophy and heart failure in the aforementioned experimental model of knockout mice, lacking VDR [5].
7. EFFECTS OF VITAMIN D REPLACEMENT ON CARDIOVASCULAR DISEASE
Data from randomized controlled trials to assess the impact of vitamin D supplementation on cardiovascular risk are limited, particularly in relation to heavy outcomes, and have reported sometimes conflicting results. Overall, the evidence is derived from studies that addressed specific populations. In addition, some studies were limited by the small sample size, lack of uniformity in the inclusion criteria, short observation period and use of relatively low doses of vitamin D. Table 1 presents the randomized placebo-controlled trials that evaluated the effects of vitamin D supplementation on cardiovascular outcomes.
Randomized placebo-controlled trials evaluating the effects of vitamin D supplementation over cardiovascular outcomes.
| Author | N | Study population | Follow-up | Intervention | Main results |
|---|---|---|---|---|---|
| Hsia et al. [49] | 36,282 | Healthy postmenopausal women (50-79 years) | 7 years | Calcium 1.000 mg + vitamin D 400 IU vs placebo | No effect on coronary and cerebrovascular events |
| Wood et al. [50] | 305 | Healthy women (60-70 years) | 1 year | Vitamin D 400 or 1,000 IU vs placebo | Small changes in apolipoprotein B 100 levels (-1,0 mg/dl in 400 IU subgroup, -1,0 mg/dl in 1,000 IU subgroup and +0,02 mg/dl in placebo subgroup) |
| Gepneret al. [52] | 114 | Healthy women with a mean age of 64 years and serum levels of 25(OH)D between 10 and 60 ng/ml | 4 months | Vitamin D 2,500 IU vs placebo | No effect on FMD, carotid-femoral pulse wave velocity, aortic augmentation index and serum levels of hs-CRP |
| Pfeifer et al. [53] | 148 | Elderly women (mean age 74 years) with serum levels of 25(OH)D < 20 ng/ml | 8 weeks | Vitamin D 800 IU + calcium 1,200 mg vs only calcium 1,200 mg | Significant decreases in systolic blood pressure, heart rate and serum levels of PTH |
| Forman et al. [54] | 283 | Healthy black individuals with a mean age of 51 years | 3 months | Vitamin D in different dosages (1,000, 2,000 or 4,000 IU) vs placebo | Small but significant decreases in systolic blood pressure in all vitamin D subgroups |
| Mitri et al. [55] | 92 | Adult individuals (mean age 57 years) with glucose intolerance | 16 weeks | Vitamin D 2,000 IU vs calcium 800 mg | Significant increase in insulin secretion in vitamin D group |
| Witham et al. [56] | 61 | Type 2 diabetics with serum levels of 25(OH)D < 40 ng/ml | 16 weeks | Vitamin D in different single doses (100.000 ou 200.000 IU) vs placebo | Significant decreases in systolic blood pressure in both vitamin D subgroups at eight weeks analysis as well as in type B natriuretic peptide in the subgroup that was treated with vitamin D 200.000 IU at 16 weeks analysis |
| Yiu et al. [57] | 100 | Type 2 diabetics | 12 weeks | Vitamin D 5,000 IU or placebo | No significant effect on FMD neither on markers of inflammation and oxidative stress, lipid profile and glycaded hemoglobin |
| Zittermann et al. [58] | 200 | Healthy obese individuals with low serum levels of 25(OH)D (mean 12 ng/ml) | 12 months | VitaminD 83 microgram vs placebo | Significant decrease in serum levels of triglycerides and TNF-α and a small but significant increase in LDL-cholesterol levels |
| Witham et al. [61] | 105 | Elderly (≥70 years) with systolic heart failure and serum levels of 25(OH)D <20 ng/ml | 20 weeks | Vitamin D 100,000 IU vs placebo at the beginning and ten weeks after | No benefits in quality of life ( Minnesota score) and serum levels of TNF-α but significant decrease in type B natriuretic peptide |
In postmenopausal women, the findings have been quite varied and generally disappointing. In the classic Women's Health Initiative (WHI) study, which involved 36,282 postmenopausal healthy women, aged 50-79 years, randomly assigned to take 1000 mg of calcium plus 400 IU of vitamin D daily or placebo and followed for seven years, no significant difference between the treatment and placebo groups was observed for the incidence of coronary and cerebrovascular events [49]. It should be stressed, however, that besides the low doses used, deficiency of vitamin D did not constitute a criterion for inclusion in the study. In the WHI, the fact that women with higher body mass index and multiple coronary risk factors have shown a lower incidence of cardiovascular events in the active treatment group draws attention.
In a study involving 305 healthy women aged 60-70 years who received randomly 400 or 1,000 IU of vitamin D3 or placebo daily for one year to evaluate the effects of the vitamin on lipid profile, insulin resistance, inflammatory biomarkers and blood pressure, the authors observed only minor changes in the levels of apolipoprotein B100 (-1.0 mg/dl in the 400 IU group, -1.0 mg/dl in the 1,000 IU group and +0.02 mg/dl in the placebo group), that, although significant, were considered clinically irrelevant [50]. However, it must be stressed, as a limitation, that, in this study, vitamin D deficiency also did not constitute a criterion for inclusion.
Likewise, Gannagé-Yared et al. [51], evaluating a set of only 47 healthy postmenopausal women, regarding the effects of a 12-week course of 800 IU of vitamin D associated to 1,000 mg of calcium per day on the inflammatory profile, pancreatic function and lipid parameters, did not observe any significant change in serum levels of interleukin 6, tumor necrosis factor-α, hs-CRP, insulin, triglycerides, HDL and LDL-cholesterol. We emphasize that in this study, vitamin D deficiency was also considered an inclusion criterion.
Gepner et al. [52], in prospective, randomized, double-blind study involving 114 women with a mean age of 64 years, with serum 25(OH)D between 10 and 60 ng/ml, in which the effects of oral administration of 2,500 IU of vitamin D3 per day for four months on cardiovascular risk markers were compared with placebo, also did not show any significant difference between the two groups regarding changes in FMD, carotid-femoral pulse wave velocity, aortic augmentation index or serum levels of hs-CRP.
However, in a study involving 148 elderly women (mean age 74 years) with documented hypovitaminosis D [serum 25(OH)D<20 ng/ml], randomized to receive for eight weeks oral supplementation of 800 IU of vitamin D3 plus 1,200 mg of calcium or just 1,200 mg of calcium daily, Pfeifer et al. [53] reported significant decreases in systolic blood pressure, heart rate and serum levels of PTH in the participants of the first group.
Favorable effects of oral vitamin D supplementation on blood pressure were also reported by Forman et al. [54] that evaluated healthy blacks, a population with a known high prevalence of hypovitaminosis D. In this study, 283 individuals with a mean age of 51 years, almost half in use of antihypertensive drugs, were randomized in a double-blind fashion to receive oral vitamin D3 in different doses (1,000, 2,000 or 4,000 IU per day) or placebo for three months. In the analysis of three months, the authors found small but significant changes in systolic blood pressure (on average, +1.7 mmHg in the placebo subgroup and -0.66, -3.5 and -4.0 mmHg in the three active treatment subgroups, respectively).
Some beneficial effects of vitamin D have also been reported in diabetic and glucose intolerant patients. In the Calcium and Vitamin D for Diabetes Mellitus (CaDDM) study, conducted by Mitri et al. [55], in which 92 adults (mean age 57 years) with glucose intolerance were randomized in a double-blind fashion to receive 2,000 IU of vitamin D or 800 mg calcium per day for 16 weeks, the authors observed an increased insulin secretion in the vitamin D group and a decrease in the control group, with statistically significant difference, as well as a smaller increase in glycated hemoglobin in the vitamin D group, compared to the control group, with borderline statistical significance.
In order to investigate the effects of high doses of vitamin D3 orally on vascular health and glycemic control in type 2 diabetic patients, Witham et al. [56] randomized, in a double-blind fashion, 61 subjects with serum 25(OH)D<40 ng/ml to receive a single dose of placebo or different doses of vitamin D3 (100,000 or 200,000 IU). In the analyses of eight and 16 weeks, the authors observed no significant differences between the groups regarding endothelial function assessed by FMD, insulin resistance and glycated hemoglobin levels. However, they found significant reductions, compared to placebo, in systolic blood pressure in both groups that received vitamin D, in the analysis of eight weeks, as well as in B-type natriuretic peptide in the group that received 200,000 IU, in the analysis of 16 weeks.
And also in type 2 diabetics, Yiu et al. [57] showed no significant effect of high-dose vitamin D supplementation on vascular function and inflammatory parameters. In this study, 100 subjects were randomized in a double-blind fashion to take vitamin D orally at a dose of 5000 IU/day or placebo for 12 weeks. In the end, the authors did not show any significant effect of vitamin D supplementation on endothelial function, assessed by FMD, serum levels of endothelial progenitor cells and brachial-ankle pulse wave velocity, or on biomarkers of inflammation and oxidative stress, lipid profile and glycated hemoglobin.
Some benefits of oral supplementation of vitamin D have been demonstrated in obese individuals. In 200 healthy overweight individuals with low serum 25(OH)D (mean 12 ng/ml) participants in a weight control program, randomized in a double-blind fashion to receive vitamin D (83 micrograms/day) or placebo for 12 months, Zittermann et al. [58] found that vitamin supplementation was associated with significant reductions in serum triglyceride levels and the inflammatory marker tumor necrosis factor-α, although it has promoted a small but significant increase in LDL-cholesterol.
In some studies, a favorable effect of vitamin D supplementation on endothelial function was demonstrated. In the trial conducted by Tarcin et al. [13], involving 23 asymptomatic individuals with severe deficiency of 25(OH)D (serum levels below 10 ng/ml), it was observed, after vitamin D replacement in the form of 300,000 IU intramuscularly monthly for three months, a significant improvement of endothelial function, reflected by an increase in FMD. Similar results, also using high doses of vitamin D, were observed by Harris et al. [59] in African Americans and by Witham et al. [60] in stroke patients.
The effects of vitamin D supplementation on functional capacity and quality of life in patients with HF were assessed by Witham et al. [61]. In the study, in which 105 elderly patients (≥ 70 years) with systolic heart failure and vitamin D deficiency [25(OH)D<20 ng/ml] were randomized in a double-blind fashion to receive orally 100,000 IU of vitamin D2 or placebo at the beginning and 10 weeks later, no benefit in favor of the active treatment group was observed in the analyses of 10 and 20 weeks, with respect to various parameters evaluated such as six-minute walk test, quality of life according to Minnesota Living with Heart Failure Questionnaire score and serum levels of tumor necrosis factor-α. However, the authors noted, at 10 weeks, a decrease in serum atrial natriuretic peptide in the vitamin D group and an increase in the placebo group, with significant difference between groups.
The effects of oral supplementation of vitamin D have also been tested on 123 heart failure patients randomized to receive 50 micrograms of vitamin D (2000 IU) plus 500 mg of calcium or placebo plus 500 mg of calcium per day for nine months. The survival rate was not different between the two groups after 15 months of follow-up but a more favorable inflammatory profile was observed in the group receiving vitamin D, translated by higher levels of interleukin 10, an anti-inflammatory cytokine, and lower levels of tumor necrosis factor-α, which has an inflammatory action [62].
Results even more favorable with oral vitamin D supplementation in patients with HF were recently reported by Amin et al. [63]. In the study, 94 subjects with serum levels of 25(OH)D below normal (<30 ng/ml) were given oral supplementation of vitamin D3 for four months, being 50,000 IU per week for eight weeks and then 50,000 IU monthly for the following two months. In the final evaluation, the authors observed a significant reduction in serum levels of brain natriuretic peptide (pro-BNP) and hs-CRP, as well as significant improvement in functional class and increase in 6-minute walk distance.
Through a case-control evaluation of participants of the EURODIAB Study [42], the impact of vitamin D supplementation during infancy on the risk of developing type 1 diabetes in the future was investigated. Analyzing data from 820 cases and 2,335 controls, the authors found that vitamin D supplementation in infancy was associated with a significant decrease in the incidences of type 1 diabetes, even after adjustment of several potential confounders, with an estimated odd ratio of 0.67, which reinforces the possible immunomodulatory effect and protective role of the vitamin against the development of the disease in susceptible individuals.
Unfortunately, the large multicenter study Thiazolidinedione Intervention with Vitamin D Evaluation (TIDE), randomized, double-blind, placebo-controlled, designed to include 16,000 diabetics in 33 countries to be followed for more than five years, in which 1,221 individuals came to be assigned to receive 1,000 IU per day of vitamin D or placebo, with the primary objective of evaluating the effects of the vitamin on all-cause mortality and incidence of cancer, had to be stopped early for internal regulatory reasons, without reaching the goals, being observed, however, comparable incidence of adverse events in the two groups [64].
Finally, it is worth noting that vitamin D supplementation is considered safe: under natural conditions, a full body exposure to sunlight, for example, is able to induce rapidly (<20 minutes) to the synthesis of the equivalent of more than 10,000 UI without any known adverse effects, apart from the possible harm to the skin, i.e., does not cause intoxication, because excess vitamin D3 is simply converted into inactive products [65]. Indeed, accumulating evidence has shown that prolonged intake of 10,000 IU/day (or 250 micrograms) of vitamin D3 causes, in general, no risk of adverse effects, so this dose can be considered totally safe. Thus, vitamin D intoxication is extremely rare, occurring only upon accidental or intentional ingestion of excessively high doses, more than 50,000 IU per day, in which serum levels of 25(OH)D may exceed 150 ng/ml and cause hypercalcemia and hyperphosphatemia [65].
However, the dose of oral supplementation of vitamin D3 (cholecalciferol) required in individuals who live without adequate sunlight exposure also has been a matter of debate. The Institute of Medicine recommends, as an adequate intake, daily doses of 200 IU for children and adults up to 50,400 IU for adults 51-70 years and 600 IU for those over 70 years. However, most experts agree that, without adequate sun exposure, children and adults require approximately 800 to 1,000 IU per day. In individuals with proven vitamin D deficiency, Holick [65] has recommended doses even higher: 50,000 IU per week for eight weeks, followed by half this dose indefinitely.
8. FINAL CONSIDERATIONS
Vitamin D appears to play an important role in cardiovascular health. Numerous studies have shown, as demonstrated above, strong independent association between hypovitaminosis D and cardiometabolic risk. Increasing evidence suggests that the effects on the cardiovascular system may stem from both indirect actions, via modulation of known risk factors, and direct actions on cardiac and vascular cells. Although there is still need for further studies, the potential importance of vitamin D deficiency as an emerging major public health problem of global proportions, with important implications for cardiovascular morbidity and mortality is undeniable. Specifically in relation to CVD, hypovitaminosis D may be of particular importance, considering, on one hand, the high prevalence of both conditions around the world and, on the other, the possibility of its prevention and correction in a simple way.
However, we still lack thorough evidence, based on large randomized placebo-controlled studies using higher doses of vitamin D, with longer-term follow-up and with adequate statistical power to assess heavy outcomes, so you can establish definitively the role of vitamin D, especially in its oral form, in the prevention and treatment of CVD.As seen, many published studies have involved small samples, specific populations and there is no uniformity regarding the inclusion criteria or the dose of vitamin D administered and duration of treatment, and often vitamin D has been administered together with calcium. So, further studies to evaluate primarily the role of vitamin D and to establish its appropriate dose in this context are needed. We believe, however, that the already published data linking vitamin D deficiency and numerous health problems, not just cardiovascular disease, and pointing to the multiple benefits of its replacement, which can be made in a simple and safe way, argue strongly in favor of prevention and treatment of hypovitaminosis D in order to reduce cardiovascular morbidity and mortality, despite the lack of definitive studies in this area, as already explained.
CONFLICT OF INTEREST
The authors state that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
The authors thank Ribamar Martins and Hudson Santos, from the Open University of Brazilian Health System (UNA-SUS), São Luís, Maranhão, Brazil, for the illustrations presented in this article.


