All published articles of this journal are available on ScienceDirect.
Comparative Effects of Preoperative Angiotensin-converting Enzyme In-hibitor, Statin and Beta-blocker Treatment on Human Internal Mammary Artery Reactivity in Patients with Coronary Artery Disease: A Pilot Study
Abstract
Purpose:
We investigated the effect of angiotensin-converting enzyme (ACE)- inhibitor, statin, and beta-blocker usage before coronary bypass surgery (CABG) on vascular reactivity of the internal mammary artery (IMA).
Methods:
Patients, who underwent elective CABG were evaluated. Samples of IMA obtained from 22 patients were divided into 4 groups in respect of drugs used by patients before bypass surgery (control group, ACE inhibitor + statin group, ACE inhibitor + statin + beta-blocker group, and ACE inhibitor + beta-blocker group). The discarded, distal end section of IMA was carefully removed, and the vasoreactivity of IMA rings was evaluated in vitro using an organ chamber. Smooth muscle contractile function was tested on artery segments exposed to 10-80 mM KCl and norepinephrine. The endothelial function of IMA rings was assessed with acetylcholine (ACh) and bradykinin, while endothelium-independent vasorelaxation was evaluated by sodium nitroprusside (SNP).
Results:
Both ACh and bradykinin caused concentration-dependent relaxation in endothelium-intact IMA rings. However, the maximal effect produced by endothelium-dependent agents in all treatment groups was more prominent when compared with the control group. There was no significant difference in the endothelium-dependent relaxation response of IMA between ACE inhibitor + statin, ACE inhibitor + beta-blocker and ACE inhibitor + statin + beta-blocker groups. The vasodilatory potency of SNP was similar in all groups. Similarly, contractile response to KCl or norepinephrine was not significantly different between groups.
Conclusion:
Use of ACE inhibitors and statins before bypass surgery may influence IMA vasoreactivity by improving endothelial control of vascular tone.
INTRODUCTION
The normal endothelium plays a key role in the local regulation of the vascular tone by producing and releasing vasodilators, as well as vasoconstrictors [1, 2]. Several studies have demonstrated the importance of preserving the function of endothelium in autogenous arterial grafts [3, 4]. Endothelium plays an important role in the grafts used in coronary artery bypass grafting (CABG) [5]. Endothelial dysfunction, which is commonly associated with an inability of the endothelium to initiate vasodilation in response to vasodilatory stimuli such as acethylcholine or shear stress caused by decreased nitric oxide (NO) biovailability in the vessel wall [6]. Endothelial dysfunction is thought to be an early physiologic event in the development of atherosclerosis in human coronary arteries [7], and could be associated with the prognosis of the disease following CABG [8, 9]. The different tendency of arterial grafts to go into vasospasm may also be related to the different endothelial function in grafts used for CABG [10]. Hence, impaired endothelial function may adversely affect the function and patency of internal mammary artery (IMA) coronary bypass grafts.
Treatments able to reverse coronary endothelial function might benefit graft patency. Changes in vasoreactivity of IMA grafts depending on used cardiovascular drugs may have some relevance in the clinical setting. Most pharmacological interventions to improve the function of endothelium targeted the risk factors linked to endothelial dysfunction such as hypertension and dyslipidaemia. Nevertheless, several pharmacological agents have been suggested to achieve vascular protection through different mechanisms that go beyond their primary therapeutic actions (e.g., angiotensin-converting enzyme (ACE)-inhibitors, statins and third generation of beta-blockers) [11, 12].
The IMA is widely used as a conduit for CABG, can be readily obtained, and serves as a useful model to study the effects of pharmacologic agents on human vasculature. Despite the widespread use of the IMA in CABG, there is a lack of comparative studies on IMA endothelial-dependent and independent functions in patients who have received cardiovascular drugs preoperatively. Since cardiovascular drugs are now widely used, their effect on endothelial function is likely to be of importance. In the light of these explanations, the aim of this study was to evaluate the effects of preoperative ACE inhibitor, statin and beta-blocker treatment on IMA vasoreactivity in patients undergoing CABG.
MATERIALS AND METHODOLOGY
Study Protocol
This study was performed in Akdeniz University Hospital with totally 22 patients who underwent isolated CABG. Data were retrospectively collected from patient records between January 2005 and June 2005, and samples of these patients were divided into following groups in respect of drugs used by patients before bypass surgery: 1) Control group, 2) ACE inhibitor + statin group, 3) ACE inhibitor + statin + beta-blocker group, and 4) ACE inhibitor + beta-blocker group. The patients in control group did not take ACE inhibitor, statin or beta-blocker therapy at least 2 months preoperatively. ACE inhibitor + statin group took statin plus ACE inhibitor therapy daily for at least 6 months preoperatively, ACE inhibitor + statin + beta-blocker group took all of these drugs daily for at least 6 months preoperatively and those in ACE inhibitor + beta-blocker group took ACE inhibitor and beta-adrenoceptor antagonist therapy daily at least 6 months preoperatively. Diabetic patients and patients with renal or hepatic impairment, congestive heart failure, active inflammatory or immunomodulatory diseases, a history of myocardial infarction in the past 6 months, and pregnant women were excluded. Baseline characteristics of these patients were listed in Table 1. Segments of left IMA were collected from 22 patients undergoing CABG. The discarded distal end was carefully removed and placed in Krebs solution of the following composition (mM: NaCl 118, KCl 5, NaHCO3 25, KH2PO4 1.0, MgSO4 1.2, CaCl2 2.5, and glucose 11.2). The time delay between vessel harvest and preparation was less than 30 min. The vessels were transferred to the laboratory and then cleaned of the connective tissue. The IMA segments were cut into 3-mm rings; 2 to 4 rings were obtained from each vessel.
Baseline Characteristics of the Patients
| Characteristic | Control | ACEI+STAT | ACEI+BB | ACEI+STAT+BB |
|---|---|---|---|---|
| Age (years) | 62.8 ± 2.4 | 64.2 ± 2.9 | 63.0 ± 2.6 | 65.8 ± 2.3 |
| Women | 1 | 1 | 1 | 1 |
| Current smoking | 3 | 4 | 3 | 3 |
| Hypertension | 3 | 6 | 6 | 6 |
| Hypercholesterolemia | 3 | 6 | 4 | 6 |
| Pre-admission drugs | ||||
| Statins | 0 | 6 | 0 | 6 |
| Beta-blockers | 0 | 0 | 6 | 6 |
| ACE inhibitors | 0 | 6 | 6 | 6 |
Data are median ± SEM or number of patients.
ACEI: Angiotensin-converting enzyme (ACE) inhibitor., STAT: Statin., BB: beta-blocker. The patients in control group did not take ACE inhibitor., statin or beta-blocker therapy for at least 2 months preoperatively. The patients in treatment groups took ACE inhibitor., statin or beta-blocker therapy daily for at least 6 months preoperatively.
Blood Glucose (mg/dl)., Cholesterol (mg/dl) and Triglyceride (mg/dl) Levels in All Groups
| Glucose | Total-C | LDL-C | HDL-C | TG | |
|---|---|---|---|---|---|
| Control | 125 ± 12 | 164 ± 29 | 91 ± 32 | 41 ± 4 | 154 ± 41 |
| ACEI + BB | 115 ± 16 | 156 ± 25 | 103 ± 18 | 38 ± 3 | 143 ± 10 |
| ACEI + STAT | 114 ± 21 | 163 ± 15 | 78 ± 6 | 50 ± 7 | 137 ± 35 |
| ACEI + BB + STAT | 159 ± 26 | 204 ± 22 | 144 ± 21 | 33 ± 3 | 138 ± 20 |
All values are expressed as mean ± SEM, n = 4-6 for all groups. Total-C: total cholesterol, LDL-C: LDL cholesterol, HDL-C:HDL cholesterol, TG: triglyceride, ACEI: Angiotensin-converting enzyme inhibitor, BB: beta-blocker, STAT: statin.
pD2 (-log EC50) Values for Norepinephrine (NE)., Acetylcholine (ACh)., Bradykinin (BDK) and Sodium Nitroprusside (SNP) of Human Internal Mammary Artery in All Groups.
| Control | ACEI+STAT | ACEI+BB | ACEI+STAT+BB | |
|---|---|---|---|---|
| NE | 6.52 ± 0.06 | 6.84 ± 0.16 | 7.02 ± 0.03* | 6.73 ± 0.05 |
| ACh | 7.21 ± 0.23 | 7.17 ± 0.38 | 7.53 ± 0.27 | 6.82 ± 0.15 |
| BDK | 7.09 ± 0.22 | 8.76 ± 0.42* | 8.50 ± 0.52 | 7.69 ± 0.34 |
| SNP | 8.01 ± 0.19 | 7.84 ± 0.23 | 7.83 ± 0.16 | 7.94 ± 0.19 |
ACEI: Angiotensin-converting enzyme inhibitor., STAT: Statin., BB: beta-blocker. *p<0.05 when compared with the control group.
The rings were carefully suspended by 2 stainless steel clips passed through the vessel lumen in 20 ml organ baths filled with Krebs solution maintened at 37°C gassed with 95% O2 and 5% CO2 to obtain a pH of 7.4. Isometric tension was continuously measured with an isometric force transducer (FDT10-A, Commat Ltd., Ankara, Turkey), connected to a computer based data acquisition system (TDA 97, Commat Ltd., Ankara, Turkey). Two g of tension was progressively applied to each IMA ring and allowed to equilibrate for 60 min. After the equilibration period, active endothelial function was confirmed by acetylcholine-induced vasodilation. Then, the rings were assessed with respect to their ability to vasodilate or vasoconstrict in response to a range of vasoactive mediators. Smooth muscle contractile function was tested by 10-80 mM KCl and norepinephrine (NE). In the first set of experiments, 10-80 mM KCl responses were performed in segments obtained from IMA. Then, tissues were challenged with NE (10-8-10-5 M) by addition of increasing concentrations of agonist to the baths in a cumulative manner and the isometric tension devoloped by the tissue recorded. The tissue response was allowed to reach a stable plateau (2-4 min) before each successive concentration of the agonist was added. In a separete of experiments, increasing concentrations of acetylcholine (ACh, 10-8-10-4 M), bradykinin (10-11-10-6 M), and sodium nitroprusside (SNP, 10-10-10-5 M) were assessed on IMA segments precontracted with 10-6 M NE, and the ability of the IMA rings to relax was determined. The endothelial function was assessed with ACh and bradykinin, while endothelium-independent vasorelaxation was evaluated by SNP.
Materials
NE, ACh, SNP, bradykinin and the salts for the Krebs solution were purchased from Sigma Chemical (St. Louis, MO). All drugs were prepared daily during experiments, and were dissolved in distilled water before use.
Statistical Analysis
All values are expressed as mean ± SEM. The curves were analyzed by using a computer program (Graph Pad Prism version 3.0 for Windows, GraphPad Software, San Diego, CA). Changes in tension in response to vasoactive agents are normalized to the maximal response induced by 80 mM KCl and are expressed as percent changes. Responses to ACh, SNP, and bradykinin are expressed as percentages of the reversal of the tension developed in response to NE. The logarithm of the concentration of agonists which elicited a 50% of maximal response (Emax) was designated as the EC50. These values were determined by regression analysis of the linear portions of the log concentration-response curves. Sensitivity was expressed as pD2 (−log EC50). Statistical analysis of the results was performed by one-way ANOVA followed by Newman-Keuls post hoc test or Student’s t-test where appropriate. A p < 0.05 was considered significant.
RESULTS
As shown in Table 1, baseline characteristics of the patiens were similar in all groups. The blood glucose and lipid levels were not significantly different between these groups (Table 2). There were no differences in baseline pre-contraction levels between the groups, and the maximum contraction achieved by NE in control IMA rings was stable for the duration of the relaxation experiments. Vascular contractions induced by either KCl or NE in IMA rings collected from patients undergoing CABG were not significantly different between these groups (Figs. 1 and 2, and Table 3). Concentration-reponse curves to NE showed no significant differences in the sensitivity among these groups except the ACE inhibitor + beta-blocker group (Table 3).
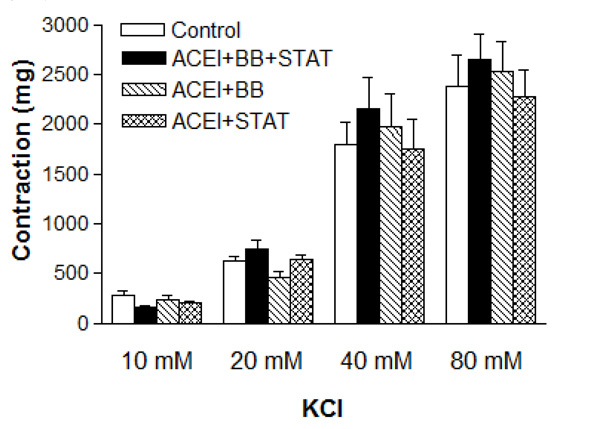
Effect of preoperative angiotensin-converting enzyme inhibitor, statin and beta-blocker treatment on contraction induced by KCl in human internal mammary artery. All values are expressed as mean ± SEM; n = 4-6 for all groups. ACEI: Angiotensin-converting enzyme inhibitors; BB: beta-blockers; STAT: statins.
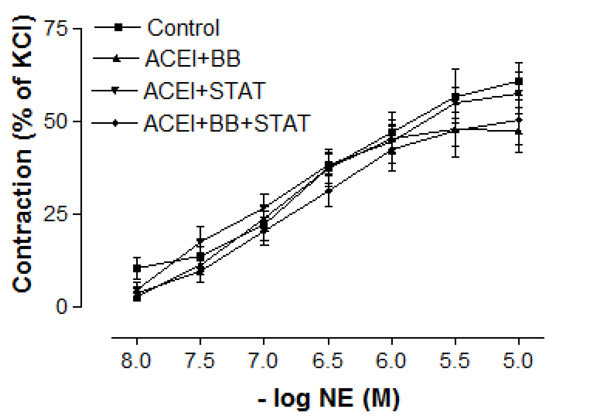
Effect of preoperative angiotensin-converting enzyme inhibitor, statin and beta-blocker treatment on contraction induced by norepinephrine in human internal mammary artery. All values are expressed as mean ± SEM; n = 4-6 for all groups. Angiotensin-converting enzyme inhibitors; BB: beta-blockers; STAT: statins.
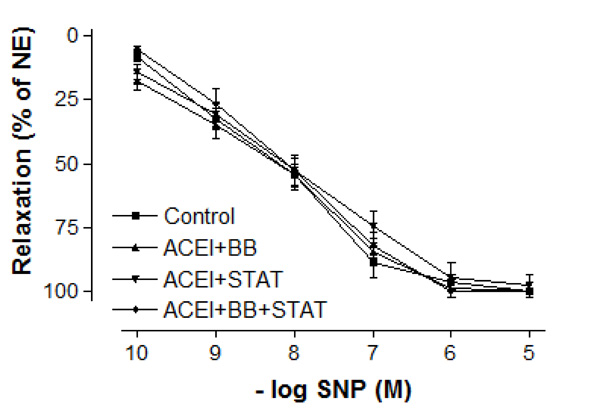
Effect of preoperative angiotensin-converting enzyme inhibitor, statin and beta-blocker treatment on relaxation responses induced by various concentration of sodium nitroprusside in human internal mammary artery. All values are expressed as mean ± SEM; n = 4-6 for all groups. Angiotensin-converting enzyme inhibitors; BB: beta-blockers; STAT: statins.
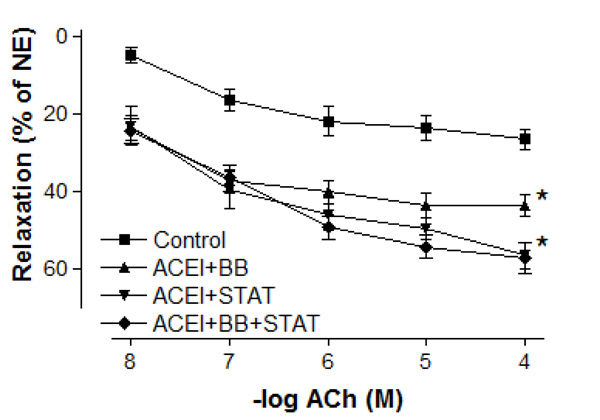
Effect of preoperative angiotensin-converting enzyme inhibitor, statin and beta-blocker treatment on relaxation responses induced by various concentration of acetylcholine in human internal mammary artery. All values are expressed as mean ± SEM; n = 4-6 for all groups. Angiotensin-converting enzyme inhibitors; BB: beta-blockers; STAT: statins.
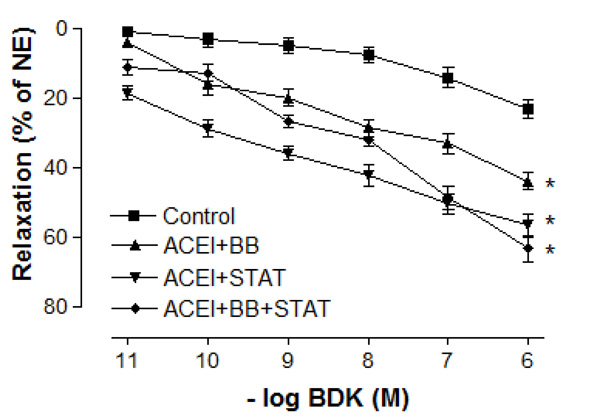
Effect of preoperative angiotensin-converting enzyme inhibitor, statin and beta-blocker treatment on relaxation responses induced by various concentration of bradykinin in human internal mammary artery. All values are expressed as mean ± SEM; n = 4-6 for all groups. Angiotensin-converting enzyme inhibitors; BB: beta-blockers; STAT: statins.
In vessel segments precontracted with 10-6 M NE, a similar concentration-dependent relaxation to the endothelium-independent agonist SNP was observed in all groups (Fig. 3). The maximum relaxation responses to SNP were also not significantly different between these groups (Fig. 33). Similarly, the sensitivities of IMA to SNP were not significantly different between control, ACE inhibitor + statin, ACE inhibitor + beta-blocker, and ACE inhibitor + statin + beta-blocker groups (Table 3).
Maximal vasodilatory effect produced by endothelium-dependent agents in all of the treatment groups was more prominent when compared with the control group (Figs. 4 and 5). Either ACE inhibitor + statin, ACE inhibitor + beta-blocker, or ACE inhibitor + statin + beta-blocker therapy markedly augmented endothelium-dependent relaxations to both ACh and bradykinin in IMA segments collected from patients undergoing CABG. Although the maximal relaxation response to ACh or bradykinin was more significant in ACE inhibitor + statin group than in ACE inhibitor + beta-blocker group, there was no significant difference in the endothelium-dependent relaxation response of IMA between ACE inhibitor + statin, ACE inhibitor + beta-blocker and ACE inhibitor + statin + beta-blocker groups. Otherwise, the sensitivities of IMA to endothelium-dependent agonists were not significantly different between these groups. As seen in Table 3, pD2 value for ACh and bradykinin was similar in all groups. The sensitivities of IMA to endothelium-dependent agonists are seen in Table 3.
DISCUSSION
The present study highlights the effectiveness of the ACE inhibitor and statin therapy in ameliorating endothelial dysfunction, as assessed by endothelium-dependent vasodilation to ACh which is mediated mainly through NO, in patients undergoing CABG. Results of the present study indicated that treatment with ACE inhibitors and statins more than 6-month period is able to improve endothelium-dependent vasodilation in the IMA rings of coronary artery patients, while beta-blockers did not provide significant additional protective effect on endothelial function.
Several pharmacological treatments may induce an alteration in the reactivity of IMA grafts through different mechanisms. ACE inhibitors have been shown to normalise endothelial function in patients with hypertension [13], chronic heart failure [14] and insulin-dependent diabetes mellitus by increasing NO-mediated endothelial function [15]. ACE inhibitors have also been shown to reduce the rate of mortality and to prevent cardiovascular events in patients with coronary artery disease [16, 17]. Nevertheless, there are still significant controversies regarding the pre-operative use of ACE inhibitors in patients undergoing CABG. Although ACE inhibitors are the most effective antihypertensive drug class in improving endothelium-dependent vasodilation in peripheral [18, 19] and coronary macrocirculation [20], at the present time, limited data are available on the effects of chronic ACE inhibitor administration on endothelial function of IMA grafts in patients undergoing CABG. In addition, effective lipid-lowering therapy have been shown to improve endothelial dysfunction in subjects with coronary heart disease [20, 21]. Importantly, 3-hydroxy-3-methyl-glutaryl coenzyme-A (HMG-CoA) reductase inhibitors, “statins”, are lipid-lowering drugs that have recently been shown to provide many benefits [22-24]. Numerous cholesterol-independent effects of statins that may limit atherosclerosis are probably related to inhibition of the geranylgeranylation of GTP-binding intracellular signaling proteins and involve: improved vasoreactivity, mostly through increased NO bioavailability; decreased expression of proinflammatory cytokines (interleukin-6, interleukin-1 beta, tumor necrosis factor alpha), C-reactive protein, chemokines, matrix metalloproteinases, and tissue factor with the subsequent inhibition of thrombin generation; reduced platelet activity; increased thrombomodulin expression; enhanced fibrinolysis, regulation of angiogenesis and immunomodulation [25]. Statins may enhance the generation of NO by increasing the expression of NO synthase (NOS) and by exerting radical scavenging properties [22, 23, 26, 27]. Clinical trials have shown that statins may reduce cardiovascular morbidity and mortality, and improve cardiovascular outcome after CABG [28-30]. However, clinical relevance of multiple protective effects induced by statins on human IMA graft vasoreactivity has not been clarified.
In the present study, the maximal contractile responses (expressed in % of KCl contraction) induced by NE in vessels obtained from human IMA were not different between these groups. Similarly, the sensitivities of IMA segments to contractile agent were not significantly different between ACE inhibitor + statin, ACE inhibitor + beta-blocker, and ACE inhibitor + statin + beta-blocker groups. Concentration-reponse curves to NE showed no significant difference in the range of contraction and sensitivity among these groups. Additionally, there was no significant differerence observed in the maximum contractile responses to 10-80 mM KCl between all groups. These results have indicated that all of these treatments were not able to produce any alteration in contractile responses of human IMA grafts. Moreover, the vasodilatory potency of SNP was similar in all groups. However, maximal vasodilatory effect induced by endothelium-dependent agents was more prominent in all of the treatment groups than that in control group. In vessel segments precontracted with NE, a similar concentration-dependent relaxation to the endothelium-dependent agonist ACh was seen in all of treatment groups. The maximum relaxation responses and sensitivities of the IMA grafts to ACh were not significantly different in these groups. Bradykinin, an other endothelium-dependent agonist, relaxed vessel segments from the human IMA in all of treatment groups with similar efficacy. Our results showed that responses of IMA rings to endothelium-dependent agents were similar in both ACE inhibitor + statin + beta-blocker group and ACE inhibitor + statin group. These results indicate that the protective effect of these drug combinations on human IMA endothelium may mainly be associated with ACE inhibitors and statins.
In addition to ACE inhibitors and statins, blockers of beta adrenergic-receptors are often applied perioperatively to maintain stable hemodynamic conditions in patients with coronary artery disease. Beta-blockers are commonly administered to patients with coronary heart disease, arrhythmias, hypertension, and some cases of cardiopathy. Among them, celiprolol has been shown to potentiate endothelium-dependent vasodilation in rat aortas [31]. Celiprolol has been previously shown in vivo to be an effective β1-receptor selective adrenoceptor antagonist and weak intrinsic sympathomimetic activity [32, 33]. Celiprolol increases endothelial NO synthase (eNOS) activity and decreases superoxide anion production in hypertensive patients [34]. Similarly, results of the previous studies indicated that carvedilol could mediate antihpertensive effects by an increase in antioxidant capacity and nebivolol through the rise in NO bioavailability [35]. Carvedilol is a non-selective beta-adrenoreceptor antagonist and an alpha1-adrenoreceptor with no intrinsic sympathomimetic activity [36]. On the other hand, nebivolol possesses β3-adrenoreceptor agonistic properties in addition to well-described, selective β1-adrenoreceptor antagonistic properties with no intrinsic sympathomimetic activity [37]. These results suggest the clinical usefulness of beta-adrenoceptor antagonists for preventing endothelial dysfunction of the CABG. Little is known, however, about its effects on human IMA vasoreactivity. In contrast, our results have shown that endothelium-dependent relaxations to both ACh and bradykinin were not significantly different between ACE inhibitor + statin + beta-blocker groups and ACE inhibitor + statin groups. By comparing with previous studies, the results of our study suggest that addition of beta-blockers to ACE inhibitor and statin therapy did not produce any additional effect on human IMA vasoreactivity. Importantly, none of these patients evaluated in the present study was treated with celiprolol, nebivolol or carvedilol. As indicated above, beta-adrenoceptor antagonists comprise a multitude of different agents, which may have additional properties exceeding the pure receptor blockade [38]. These differences as well as the mode of extracardiac action may have an impact on outcome of patients treated with β-adrenoceptor antagonists. Hence, the observed difference in beta-blocker effect on vascular endothelial function between our study and other studies may be related to the beta-blockers used in these studies.
LIMITATION OF THIS STUDY
Perhaps the most important limitation of this pilot study is that the numbers of patients and controls were relatively small. A study of 22 subjects is likely to be too small for most investigations. There are also limitations associated with the statistical analysis. Large-scale studies are needed to confirm these findings. Despite these limitations, this study may provide gather information prior to a larger study.
In conclusion, the results obtained in the present study indicate that ACE inhibitors and statins may produce a positive effect on human IMA vasoreactivity. Hence, pharmacological treatment with these drugs might be an effective intervention to prevent coronary artery reactivity. However, combination of beta-blockers to ACE inhibitors and statins did not show significant additive effects in term of endothelial function restoration.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
ACKNOWLEDGEMENT
None declared.


