All published articles of this journal are available on ScienceDirect.
Diltiazem-induced Transient Complete Atrioventricular Block in an Elderly Patient with Acute on Chronic Renal Failure
Abstract
Diltiazem is a calcium channel blocker commonly used in the treatment of various cardiovascular disorders such as hypertension, angina and supraventricular tachycardias. Metabolism occurs mainly in the liver, but a small percentage of unchanged drug and metabolites are excreted in the urine. Toxicity from this medication usually presents in the form of dysrrhytmias and heart block. Hence, we present a case of complete atrioventricular AV block in a patient receiving sustained- release diltiazem on a chronic basis. Risk factors such as advanced age and impaired renal function are dis-cussed.
INTRODUCTION
Diltiazem is a benzothiazepine calcium channel blocker that is commonly used in the treatment of hypertension, coronary artery disease and supraventricular tachycardias. It exerts its actions through inhibition of the slow voltage-gated (Type L) calcium channels in myocardial cells and vascular smooth muscle. The sinoatrial and atrioventricular nodes are particularly susceptible to its effects, with decreased automaticity [1]. It is metabolized primarily in the liver and excreted in the bile, while a small percentage of unchanged drug and some of its metabolites are cleared by the kidney. Drug accumulation in patients with renal failure has been considered to be negligible, and dose adjustment has not been routinely recommended [2,3]. We present a patient with acute on chronic renal failure who presented with complete atrioventricular (AV) block as a result of extended-release diltiazem in the context of worsening renal failure.
CASE REPORT
Our patient was an 88 year old Caucasian male with a history of Parkinson’s disease, hypertension, prior stroke and Stage 3 chronic renal failure who was brought to the Emergency Department with progressive dyspnea on exertion and profound fatigue for 48 hours. He had no prior history of presyncope, syncope or advanced conduction abnormalities, except for a longstanding first-degree heart block. His medications included sustained-release diltiazem 180 mg daily, aspirin 81 mg daily, simvastatin 40 mg daily, carbidopa/levodopa 25/100 mg twice a day, metolazone 5 mg daily and torsemide 10 mg daily. Vital signs on presentation were: oral temperature 36. 2 C, pulse of 38 beats per minute, respiratory rate of 20 breaths per minute, blood pressure of 141/78 mmHg, and oxygen saturation (SpO2) of 80% on ambient air. The patient was in moderate respiratory distress and had signs of volume overload. Laboratory data was significant for a potassium of 5.8 mmol/L, ionized calcium of 4.1 mg/dL, bicarbonate of 21 mmol/L, BUN of 134 mg/dL and creatinine of 5.0 mg/dL (patient’s baseline was 3.5 mg/dL a month earlier). ECG on admission revealed the presence of sinus rhythm with complete heart block (Fig 1); transthoracic echocardiogram showed normal left ventricular size and function, no wall motion abnormalities and dilated inferior vena cava. Aggressive diuresis was instituted, as well as non-invasive ventilatory support with BiPAP. Diltiazem was held, and he was given 1 g of intravenous calcium gluconate with little effect. Repeat ECG at 6 hours continued to demonstrate complete heart block, with occasional premature junctional complexes (Fig 2). He received an additional 2 g of intravenous calcium 12 hours after presentation; within 10 minutes after completion of the infusion, normal sinus rhythm with 1 to 1 conduction was restored (Fig. 3) and maintained thereafter.

ECG on admission revealed the presence of independent atrial and ventricular activity (sinus P waves with arrows), with a ventricu-lar rate of 41 beats per minute, compatible with complete AV block.
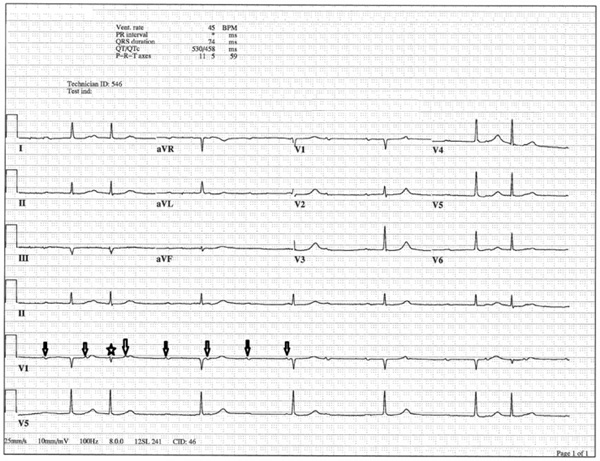
ECG after administration of 1 g of intravenous calcium. High grade AV block persisted, now with occasional premature junctional complexes (star).
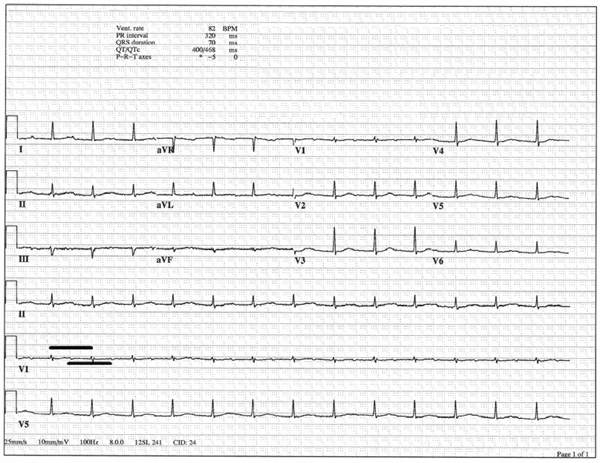
ECG after administration of an additional dose of 2 g of intravenous calcium. RR interval (bold upper line) is the same length as the PP interval (bold lower line), indicating 1 to 1 conduction with restoration of normal sinus rhythm. Notice the prolonged PR interval; the patient had an underlying first-degree heart block at baseline.
DISCUSSION
Moderate to severe overdose with diltiazem, intentional or accidental, can lead to a variety of conduction abnormalities, including sinus bradycardia, junctional rhythms and different degrees of AV block. There are rare instances in which diltiazem-naive patients present with severe dysrhythmias after a single dose of the medication [4]. It is indeed unusual in clinical practice to observe toxicity from this medication, in patients receiving chronic therapy in the proper dose range, with preserved hepatic function. Under these conditions, prior toxicity reports in the literature [4, 5] have chronic renal failure as a common element, which was true as well in our case. Limited,early pharmacokinetic studies of diltiazem in severe renal failure [2,3] concluded that there was no need for dose adjustment in these patients based on the fact that the medication and its main metabolite desacetyldiltiazem had comparable pharmacokinetic profiles in terms of peak plasma concentration, half-life and urinary excretion, as compared to patients with preserved renal function. However, it is important to emphasize that these results were derived from the administration of a single oral dose of short-acting diltiazem in a small sample of individuals with severe renal impairment. Later investigations [6] showed that patients with chronic renal failure had increased plasma levels of diltiazem and its 5 main metabolites as compared with non-renal failure patients.
Other probable contributor to the patient’s presentation was his advanced age. Andrivet et al. [4] presented a series of 10 patients, all with chronic renal failure, whom experienced complete sinus arrest during diltiazem therapy. The mean age of the cohort was 78.5 +- 3.4 years. Diltiazem elimination is slower in the elderly; elimination half-life of short-acting diltiazem increases from 3-4 hours in young subjects to up to 7 hours in aging patients [7]; other authors have reported elimination half-lives as high as 12 hours in the elderly [4]. Although the patient’s structural liver tests (transaminases, bilirubin, albumin and INR) were within normal range, hepatic metabolic reserve decreases with age.
Electrolyte imbalances, particularly hyperkalemia, can be associated with gradual depression of excitability and conduction velocity of cardiomyocites. Although effects of elevated potassium are more pronounced in Purkinje fibers and ventricular myocites, impairment of AV nodal cells can occur as well. Hyperkalemia as the sole cause of high grade AV block is usually severe [8], however, it might have played a minor adjuvant role for this patient.
There are no consensual guidelines for the management of complete AV block induced by diltiazem. Dialysis does not remove this compound. Intravenous administration of calcium has been used since the first reports of its effectiveness in the treatment of acute verapamil poisoning [9]. It has been proposed that the transient increase in serum calcium plasma concentration allows to resetting depolarization of affected myocardial cells. Doses in the range of 1- 2 g appear to be highly effective in the treatment of nodal dysfunction [4], but a precise uniform dosage remains unclear. It is interesting to note that the patient presented here regained adequate conduction with administration of 2g but not to 1 g, suggesting that a particular calcium “threshold” might need to be surpassed for the individual patient to respond.
CONCLUSIONS
Diltiazem toxicity can present with a variety of conduction abnormalities, including complete heart block. Elderly patients and those with decreased renal clearance can be at higher risk for these complications, and therefore, particular caution is advised in these patients. Further pharmacokinetic studies with sustained-release diltiazem are needed in these populations, since the available data has been obtained with immediate release formulations only. Intravenous calcium can be used in an attempt to restore normal conduction and avoid the need for pacemaker implantation.
COIFLICT OF INTERESTS
The authors confirm that this article content has no conflicts of interest.
ACKNOWLEDGEMENTS
Declared none.


