All published articles of this journal are available on ScienceDirect.
Retroperitoneal Hemorrhage as a Complication of Percutaneous Intervention: Report of 2 Cases and Review of the Literature
Abstract
Retroperitoneal hemorrhage (RPH) is an infrequent but serious complication of transfemoral percutaneous procedures. We present 2 cases and review the literature regarding the incidence, risk factors, clinical features and complications of RPH. We propose a management strategy for this problem emphasizing an anatomical based interventional approach if the patient does not stabilize with volume resuscitation.
INTRODUCTION
Retroperitoneal hemorrhage (RPH) is an infrequent but potentially fatal complication of cardiac catheterization performed using the transfemoral route [1-7]. The early history of RPH has been reviewed elsewhere [8]. The first case of RPH was reported in 1950 following lumbar anesthesia [9]. In the last decade, there has been an exponential rise in the use of interventional procedures to treat coronary artery disease [10]. Vascular complications continue to contribute to the morbidity and mortality of these procedures.
The reported incidence of RPH has ranged from 0.15-6% [2,4-6,11] with more recent studies reporting a lower incidence of approximately 0.5% [2,5]. Although the incidence appears small, considering there are 1.2 million percutaneous coronary interventions (PCIs) and 1.3 million diagnostic catheterizations performed in the United States [12], the absolute number of RPH is in thousands. RPH carries a mortality risk of 4-12% [3,5,6]. In addition, both observational and randomized trials have observed a higher 30-day mortality in patients who develop RPH after PCI [1,7]. RPH is also associated with significant morbidity, up to 5 days increase in the length of stay and need for blood transfusion [2,3,5,6].
It is known that bleeding after PCI requiring transfusion results in the addition of > $10,000 to the baseline cost of PCI [6,13]. Since the majority of the patients who develop RPH, generally require transfusion [3,6], one can understand the significant economic implications related to it.
CASE #1
A 60-year-old lady underwent diagnostic cardiac catheterization for chest pain. The right common femoral artery was used for access with a 6 French sheath. The coronary angiogram showed severe disease in the left circumflex and right coronary arteries.
In preparation for PCI, the patient received an intravenous bolus of bivalirudin (0.75 mg/Kg) and an infusion was initiated at 1.75 mg/Kg/hour. The patient became hypotensive with a systolic blood pressure (BP) of 65-70 mm Hg. A right femoral angiogram was obtained (Fig. 1) and the bivalirudin was discontinued.
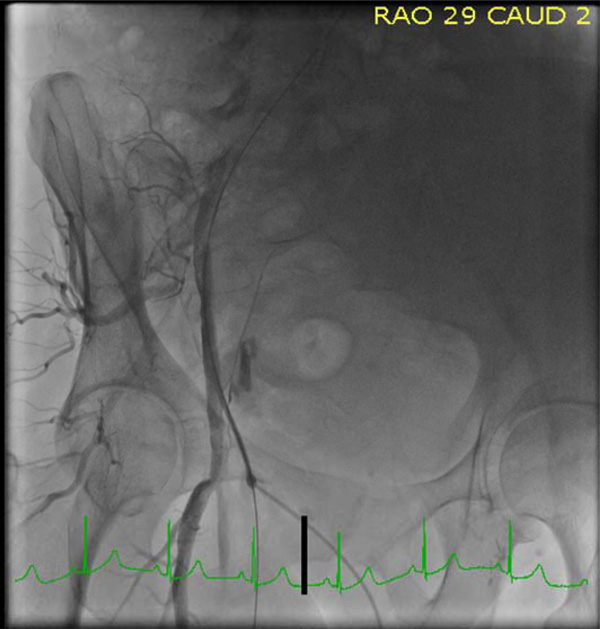
The sheath location is at upper 1/3 of the femoral head and appears above the inferior epigastric artery. Contrast is seen outside the vessel medial the sheath.

Shows an inflated balloon over the site of the retroperito-neal bleeding.

Shows the final result with the vessel sealed and no contrast outside the vessel lumen.

Shows the sheath above the inferior epigastric artery and the sheath location near the top of the femoral head.

Demonstrates a large amount of extravascular contrast with the right iliac angiogram.
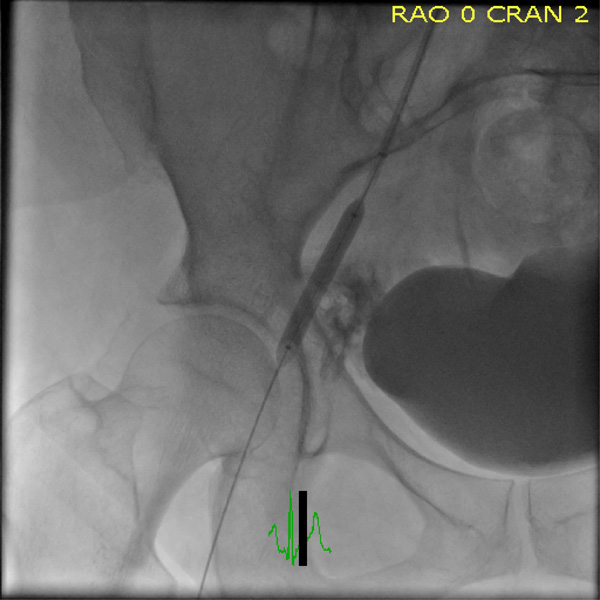
Reveals the 6x40 balloon over the site of perforation.
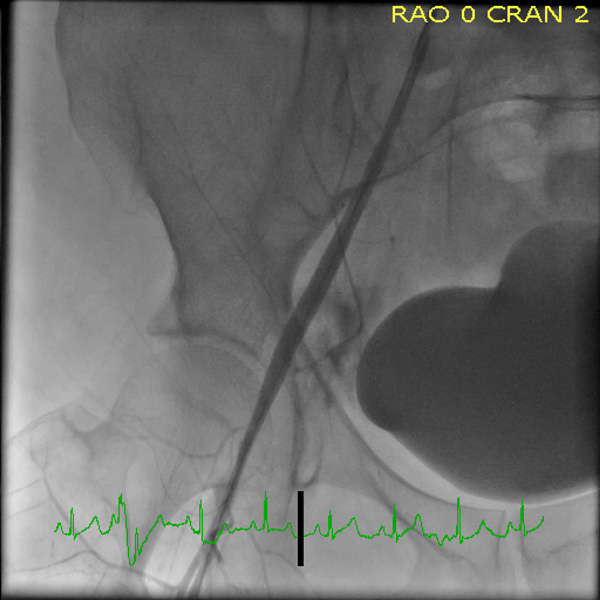
Shows the final result with the defect in the vessel sealed.
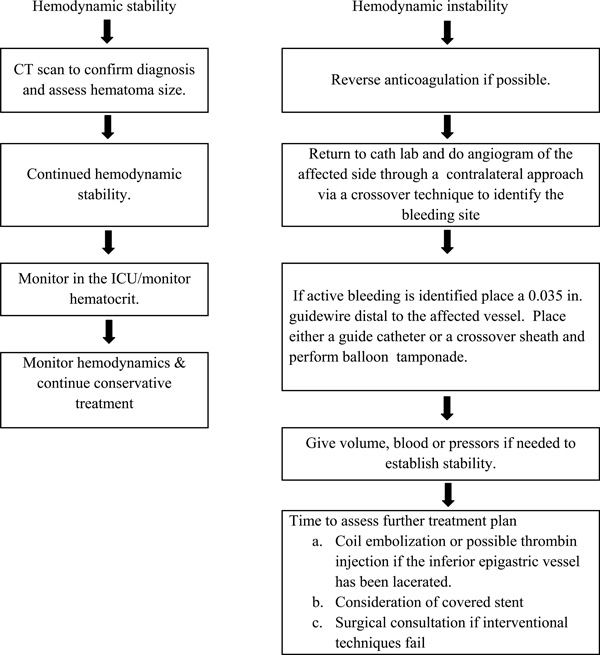
A Clinical Algorithm for Retroperitoneal Hemorrhage.
Fig. (1) shows the sheath above the inferior epigastric artery (IEA) at the upper 1/3 of the femoral head. There is clear extravasation of contrast outside the lumen of the vessel.
Immediate left femoral access was obtained, a 6 French sheath was placed in the left femoral vein and volume resuscitation was initiated. A 6 French sheath was placed in the left common femoral artery and a 6 French renal double curve Veripath guiding catheter (Abbott Vascular, Redwood City, CA) was placed over a 0.035 inch guide wire.
With the guiding catheter in the right common iliac, a 0.014 inch Balance Middle Weight (BMW) guide wire (Abbott Vascular) was placed in the right profunda femoral artery.
The BMW wire could not enter the right superficial femoral and so the wire was left in the profunda. A 5 x 13 Powersail balloon (Abbott Vascular) was positioned and inflated for 35 minutes over the perforation. Phenylephrine and normal saline were given for hemodynamic support.
Two units of packed red blood cells were given rapidly to stabilize the BP. At this point because of minimal persistent bleeding when the balloon was deflated, the 6 French sheath in the left femoral artery was exchanged for an 8 French sheath and 7 French renal double curve Veripath guiding catheter (Abbott Vascular) was placed in the right common iliac over a 0.035 inch guide wire. A 6 x 40 Fox Plus balloon (Abbott Vascular) was inflated over the perforation for 30 minutes (Fig. 2).
Once the perforation was sealed, the 0.035 inch guide wire was left in place and another 0.035 inch guide wire was placed into the abdominal aorta through the 6 French sheath in the right femoral artery. The right femoral artery was closed with a Perclose vascular closure device (VCD) (Abbott Vascular). The 6 x 40 Fox Plus balloon was then repositioned over the site of the perforation and inflated for another 10 minutes just to make sure there was no further bleeding. Once angiography showed no evidence of any further extravasation of contrast (Fig. 3), the left common femoral artery was closed with a Perclose VCD (Abbott Vascular).
The patient was hospitalized for six days and received a total of 4 units of packed cells (2 additional units after discharge from the cath lab). She was ambulatory at the time of discharge.
Two months later, she had successful PCI on the circumflex and right coronary arteries. The access was from the left common femoral artery and there were no complications.
CASE #2
A 46-year-old woman with a severe ischemic cardiomyopathy underwent cardiac catheterization because of recurrent defibrillator shocks. She was found to have severe instent restenosis in the mid circumflex and a 2.5 x 23 mm Xience stent (Abbott Vascular) was deployed with a good angiographic result. Right common femoral access was used and bivalirudin (bolus 0.75 mg/Kg and infusion 1.75 mg/Kg/hr) was given. An angiogram of the right common femoral artery was obtained (Fig. 4) and a Perclose VCD (Abbott Vascular) was deployed. The patient was sent back to the coronary care unit.
One hour after the procedure, the patient became hypotensive (systolic BP 70 mmHg) and tachycardic (heart rate: 110 beats/min). The hemoglobin concentration fell from 15.1 to 10.5 grams/deciliter. A CT scan of the abdomen showed a large RPH. The patient was transferred back to the cardiac cath lab because of persistent hypotension despite fluid resuscitation. The patient was started on dopamine 20 mcg/Kg/min on arrival to the cath lab. Left common femoral arterial access was obtained and a 6 French sheath was introduced. A Cobra 1 (5 French) [Merit Medical-South Jordan, UT] catheter was placed over a 0.035 inch guide wire into the right common iliac artery. A 0.035 inch Amplatz Stiff guide wire (260 cm)[Cook Medical, Bloomington, IN] was placed through the Cobra catheter into the right mid superficial femoral artery. The Cobra catheter and the 6 French sheath were removed and a 6 French Pinnacle Destination Sheath (Terumo Medical, Elkton, MD) was placed in the right external iliac. Angiography (Fig. 5) showed the location of the RPH. A 6 x 40 Fox Plus Balloon (Abbott Vascular) was inflated over the site of the perforation for 35 minutes (Fig. 6). There was resolution of further extravasation of blood (Fig. 7). During the procedure the dopamine was tapered to 5 mcg/Kg/min and the patient was hemodynamically stable. A Perclose VCD was used to close the left femoral arteriotomy. Two units of packed red cells were transfused.. The patient was hospitalized for 6 days and was fully ambulatory at the time of discharge.
DISCUSSION
Risk Factors
Female gender is associated with an increased risk for RPH [2,3,6,11]. The exact mechanism for this increased predisposition of bleeding in females undergoing cardiac catheterization is unclear. Females are known to have smaller diameter and shorter length common femoral arteries than men [14] which may make access more challenging. This anatomic difference may lead to a greater likelihood of multiple arterial and/or posterior wall punctures. Additionally, the majority of the females are post-menopausal age. Negative effects of lack of estrogen on arterial structure, function and integrity are known and may contribute to the increased risk of bleeding [15,16]. Both patients reported here were post-menopausal females.
Patients with low body surface area also have a smaller femoral artery diameter, resulting in arterial access issues, and therefore an increased risk of RPH [6,14].
A higher femoral arterial puncture site is an important procedure-related risk factor for RPH [2,6,17]. It is defined as a puncture above the inguinal ligament or above the middle 1/3 of the femoral head on fluoroscopy or above the IEA [2,6,17]. Puncture and sheath insertion above the inguinal ligament, and hence into the external iliac artery, may predispose to uncontrolled bleeding due to the difficulty in achieving compression of this vessel. However, it is important to realize that a lower arterial puncture does not completely eliminate the risk of RPH. In a study by Farouque et al, 45% of patients with RPH had sheath insertion in the common femoral artery, well below the presumed site of the inguinal ligament. Development of RPH in these cases can be explained by direct superior connection of the anatomical femoral sheath with the retroperitoneum allowing bleeding from the femoral vascular structures contained within the femoral sheath to reach the retroperitoneum [6].
Both cases presented here had a high stick above the IEA and above the middle 1/3 of the femoral head.
Chronic renal insufficiency (creatinine > 2 milligrams/deciliter) is associated with RPH [2,5,7].
Glycoprotein IIb/IIIa inhibitors are associated with an increased risk of RPH [5,7]. In an older study by Kent et al, aggressive post-procedural heparin use was associated with an increased risk of RPH especially in patients receiving stents [3]. However, there has been a significant decline in the degree of intra-procedure anticoagulation with heparin and post- procedure heparin infusion [7].
The data on whether VCDs (especially Angioseal) are associated with RPH is conflicting [2,5,6,18]. An increased risk of RPH with Angioseal was noted (2) in a single center, case-controlled, observational study but not in other reports [5,6]. An analysis of the National Cardiovascular Data Registry (Cath PCI) showed lower bleeding rates with VCD and bivalirudin particularly in those patients at greatest bleeding risk [19]. The discrepant findings between these nonrandomized studies of VCDs may be attributed to differences in the characteristics of the study populations, as well as the development of newer generation VCDs, improvement in technical skills and increased experience of the operators deploying the device.
In a review of 482 patients with RPH, female gender, body surface area <1.8 m², emergency procedure, history of chronic lung disease, cardiogenic shock, pre-procedure intravenous heparin, pre-procedure glycoprotein IIb/IIIa inhibitors, use of sheath size ≥ 8 French and the use of VCDs were identified as risk factors for the development of RPH [20]. The use of bivalirudin is associated with a lower risk of RPH [1,19,20].
Clinical Features
The presentation often varies and may be vague. The diagnosis can be delayed because the retroperitoneum serves as a noncompressible area where a large amount of blood can accumulate rapidly without causing obvious stigmata of an underlying expanding hematoma. Likewise there is no cutaneous bruising early in the course. In retrospective studies of patients who developed RPH following cardiac catheterization, the most common clinical features were lower abdominal pain and fullness, back or flank pain, diaphoresis [3,6] abdominal tenderness, bradycardia, hypotension and anemia [6].
Complications
Because of the concealed nature of the bleeding, the non-specific symptoms and the delayed diagnosis, RPH commonly results in hypovolemic shock, necessitating blood transfusion and an increase length of stay [3,6,21].
Abdominal compartment syndrome is a rare but serious complication of severe RPH often presenting as acute renal failure with severe abdominal pain distention causing respiratory distress and cardiovascular collapse. Emergent surgical or CT-guided drainage is required [22].
Femoral neuropathy is a well known complication of RPH, usually resulting in weakness of the iliopsoas (hip flexion) and quadriceps (knee extension) muscles and dysesthesia involving the anterior/medial thigh and medial calf [23]. The majority of cases resolve with conservative therapy but severe cases may require surgical decompression.
Management
There are no randomized trials to guide the treatment strategies for RPH and the evidence is based on small cohort series or isolated case reports. Conservative management involving intensive care unit monitoring, vigorous fluid resuscitation, blood transfusion and reversal of anticoagulation has been used as an effective strategy for the majority of patients. For patients who remain unstable in spite of aggressive resuscitation, we believe the preferred strategy is an initial percutanaeous approach while open surgery should be used only if the bleeding cannot be controlled. An interventional approach with angiography to guide the specific therapy is being increasingly utilized.
Three studies have reported on interventional treatment in patients with spontaneous RPH [24-26]. In one series,10 consecutive patients with RPH due to anticoagulation treatment were referred for an interventional approach [24]. The bleeding stopped or markedly decreased in 8 patients after embolization. A combination of coils, gelatin and/or polyvinyl alcohol was used for embolization [24].
In another study, microcoil embolization was able to stop bleeding in all five patients with recurrence of bleeding in one patient [25]. In another series, bleeding stopped in all four patients following embolization with no recurrence [26].
Mak et al. described balloon tamponade of the common femoral artery in a patient with massive RPH who had undergone diagnostic cardiac catheterization. The patient did not respond to volume resuscitation and had surgical exploration which was unsuccessful at stopping the bleeding. Subsequently prolonged balloon inflation over the bleeding site in the common femoral artery was successful in stopping the hemorrhage [27].
Samal and White have also described percutaneous approaches for the management of access site complications [28]. Balloon catheter delivery of intra-arterial thrombin has been used as well for RPH [29].
We report two patients treated by balloon tamponade who were in shock at the time of the intervention. This technique is rapidly instituted in a hemodynamically unstable patient and allows time for the anticoagulation effect to dissipate. This approach allows time to treat and improve the hemodynamics in a controlled environment and to plan a careful strategy to treat the bleeding vessel based on the anatomy producing the bleeding. The platform (6 or 7 French sheath) needed for this approach allows for the delivery of a covered stent (Atrium Medical Corporation, Hudson, New Hampshire) if the bleeding does not seal by the balloon tamponade. Likewise if there has been disruption of the IEA this technique allows time to plan a coil emolization or thrombin injection strategy if this is deemed appropriate. Vascular surgical consultation can be entertained should this conservative approach not be successful. The equipment needed to perform this procedure is present in a standard cardiac cath lab that does PCIs.. The treatment algorithm we have utilized is shown in Fig. (8).
The approach we describe with initial angiography to identify the exact bleeding site will dictate the best option for treatment. If the rapid balloon tamponade is not successful in achieving hemostasis then the bleeding site will determine the next percutaneous intervention. If the bleeding site is in the IEA then a delivery catheter can be placed in this vessel over an 0.014 inch guide wire. Depending on the vessel size either local thrombin injection or if the vessel is large enough then microcoil emolization can be undertaken. If the bleeding site is in the external iliac artery above the femoral head then a covered stent can be placed taking care to avoid a bending point. Another issue with placing a covered stent is appropriate sizing. When the patient has received vasopressors for hemodynamic support there is frequently vasospasm of the common femoral and external iliac vessels as seen in our second case. This “shrinkage” of the vessels may make the stent sizing problematic.
CONCLUSION
RPH is an infrequent but serious complication of transfemoral catheterization procedures and is associated with significant morbidity and mortality. While there has been a decline in the incidence of major bleeding in the last decade, it still remains a significant problem [7,30]. Risk factors for RPH include low body weight, female gender, emergency procedure, pre and post procedure heparin, pre-procedure IIb/IIIa inhibitors, and arterial access above the mid femoral head. Bivalirudin is associated with a lower bleeding risk.
We present a treatment algorithm that is initiated by aggressive volume resuscitation and careful monitoring of the hemodynamics. If the patient remains unstable, then an interventional approach guided by the anatomical abnormality producing the RPH is indicated. Surgical management should be considered only when the interventional approach has been unsuccessful.
CONFLICTS OF INTEREST
The authors report no financial relationships or conflicts of interest regarding the content herein.
ACKNOWLEDGEMENT
Pam Gilbert-Misner and Charles Townley for their superb skills in the cardiac catheterization laboratory.


