All published articles of this journal are available on ScienceDirect.
Actual Role of Platelet Glycoprotein IIb/IIIa Receptor Inhibitors as Adjunctive Pharmacological Therapy to Primary Angioplasty in Acute Myocardial Infarction: In the Light of Recent Randomized Trials and Observational Studies with Bivalirudin
Abstract
Strategies for preventing ischemic complications during percutaneous coronary interventions (PCI) in the setting of acute myocardial infarction (AMI) have focused on the platelet surface-membrane glycoprotein (GP) IIb/IIIa receptor. The platelet GP IIb/IIIa receptor inhibitors, by blocking the final common pathway of platelet aggregation, have become a breakthrough in the management of acute coronary syndromes. Current adjuvant pharmacological therapy of AMI with aspirin, clopidogrel, unfractionated heparin (UH), and platelet GP IIb/IIIa inhibitors provides useful therapeutic benefits. Although the use of more potent antithrombin and antiplatelet agents during PCI in AMI has reduced the rate of ischemic complications, in parallel, the rate of bleeding has increased. Several studies have reported an association between bleeding after PCI and an increase in morbidity and mortality. Therefore, investigational studies have focused in pharmacological agents that would reduce bleeding complications without compromising the rate of major adverse cardiovascular events. Based on the results of several randomized trials, abciximab with UH, aspirin and clopidogrel have become a standard adjunctive therapy with primary PCI for AMI. However, some of the trials were done before the use of stents and the widespread use of thienopyridines. In addition, GP IIb/IIIa inhibitors use have been associated with thrombocytopenia, high rates of bleeding, and the need for transfusions, which increase costs, length of hospital stay, and mortality. On the other hand, in the stent era, bivalirudin, a semi-synthetic direct thrombin inhibitor, has recently been shown to provide similar efficacy with less bleeding compared with unfractionated heparin plus platelet GP IIb/IIIa inhibitors in AMI patients treated with primary PCI. The impressive results of this recent randomized trial and other observational studies make a strong argument for the use of bivalirudin rather than heparin plus GP IIb/IIIa inhibitors for the great majority of patients with AMI treated with primary PCI. However, some controversial results and limitations in the studies with bivalirudin exert some doubts in the future widespread use of this drug.
INTRODUCTION
The prevalence of patients who have survived acute myocardial infarction (AMI) is estimated at 15 million. These patients are at high risk to develop additional acute events. The pathophysiology of acute coronary syndromes is characterized by disruption of atherosclerotic plaques, activation and aggregation of platelets, and formation of an arterial thrombus [1]. Thrombus formation can result in either transient or persistent occlusion. Common antiplatelet agents such as aspirin and clopidogrel, and, in part, antithrombin agents such as heparin, act by inhibiting different pathways of platelet activation. This may account for the limited ability of these agents to prevent platelet aggregation, since other avenues of activation are left undisturbed [2]. Heparin only inhibits thrombin-induced platelet aggregation but may stimulate aggregation initiated by other agonists [3, 4].
The critical role of glycoprotein (GP) IIb/IIIa receptors as final common pathway for platelet aggregation suggests the possibility that antagonize this receptor may be the most effective for preventing platelet aggregation and, therefore, thrombosis. Regardless of the stimulus for platelet activation, GP IIb/IIIa antagonists inhibit thrombosis by preventing fibrinogen from binding to the platelet GP IIb/IIIa receptor, the final common pathway of platelet aggregation [5-8]. Platelets are known to play an important role in the pathogenesis of acute coronary syndromes [9-12]. However, the high levels of platelet inhibition attainable with GP IIb/IIIa antagonists have failed to dramatically improve clinical outcomes outside the scenario of percutaneous coronary intervention (PCI). On the other hand, despite the success of abciximab in preventing ischemic events after PCI, the use of intravenous, small-molecule GP IIb/IIIa antagonist, and the intention to broaden the clinical indication have produced varied results. Mechanisms contributing to these heterogeneous outcomes may include the possibility of prothrombotic events, as well as, normal variation in platelet or receptor number, differences in receptor activity, and interpatient variation in pharmacological dose response. Furthermore, the intention to broaden the clinical benefits obtained with endovenous GP IIbIIIa inhibitors to oral GP IIbIIIa inhibitors as adjuvant therapy of PCI failed to reach clinical relevance; therefore, they were abandoned from this therapeutic scenario.
Based on the results of several randomized trials, abciximab with unfractionated heparin (UH) has become a standard adjunctive therapy with primary PCI for AMI, and GP IIb/IIIa inhibitors are used in the great majority of patients treated with primary PCI [13]. However, some of the trials were done before the use of stents and the widespread use of thienopyridines. In addition, GP IIb/IIIa inhibitors use have been associated with thrombocytopenia, high rates of bleeding, and the need for transfusions, which increase costs, length of hospital stay and mortality. On the other hand, in the stent era, bivalirudin, a semi-synthetic direct thrombin inhibitor, has recently been shown to provide similar efficacy with less bleeding compared with UH plus platelet GP IIb/IIIa inhibitors in elective PCI, in PCI for non-ST elevation acute coronary syndromes, and in AMI treated with primary PCI [9–13]. The results of these recent randomized trials and other observational studies make a strong argument for the use of bivalirudin rather than UH plus GP IIb/IIIa inhibitors for the great majority of patients with AMI treated with primary PCI. However, some controversial results and limitations in the studies with bivalirudin exert some doubts in the future widespread use of this drug. Therefore, this review will emphasize the actual role of these platelet GP IIb/IIIa inhibitor agents in the stent and thienopyridine era, in the light of recent randomized trials and observational studies with bivalirudin in the adjunctive therapy of AMI in the setting of primary PCI.
ROLE OF PLATELETS IN AMI
Thrombosis is often regarded as the pathologic extension of the normal haemostatic process. Under normal physiologic conditions, platelets are essencially inert; their adhesion to the subendothelial matrix is prevented by an intact vascular wall. The chief function of platelets is to coordinate with vascular endothelium and soluble plasma factors in the haemostatic process [6]. In response to vessel trauma, platelets spontaneously adhere to newly exposed adhesive proteins forming a protective monolayer of cells. These platelets are activated very rapidly by agonists such as thrombin, collagen, adenosine diphosphate, and thromboxane A2, causing morphologic changes and release of materials from stored vesicles. The constituents of the granules are involved mainly in the further activation of more platelets and the propagation of the haemostatic process [14-17].
Plaque rupture occurs when the fibrous cap of the plaque is disrupted by physical stresses and either tears or is removed completely. When the endothelial intima is interrumpted, substances such as collagen, von Willebrand factor, fibronectin or vitronectin are exposed to circulating platelets and plasma coagulation factors [16, 17]. This process of platelet adhesion forms a monolayer of cells over the endothelial lesion and specific agonists induce platelet vesicle secretion and aggregation. The final common pathway of all agonist-receptor interactions is the activation of a specific receptor responsible for platelet aggregation. This receptor, the GP IIb/IIIa is active only after the platelet has been stimulated and consequently after its intracellular calcium concentration has increased. The GP IIbIIIa is found only on cells of megakaryocytic origin and has a high affinity for the tripeptide sequence arginine-glycine-aspartic acid (RGD), which is found in fibrinogen, von Willebrand factor, fibronectin and vitronectin [14, 15]. Primarily because of its high concentration in the plasma, fibrinogen is the primary polypeptide involved in platelet aggregation. Because fibrinogen is a divalent dimer, it is able to link adjacent platelets. The presence of atherosclerotic vascular disease highly correlates with the development of platelet thrombi. Once formed, the thrombus may partially or totally occlude a vessel, resulting in alteration of blood flow and tissue ischemia or necrosis. The atherosclerotic process involves the primary progression of growth of multifocal intimal lesions from fatty streaks to fibrous plaque. Lipid-filled foam cells invade the smooth muscle-collagen cap consequently reducing its strength. Advanced plaques may also contain a sizable pool of extracellular cholesterol within the plaque’s core. These weak, yellow plaques with high lipid content are particularly inclined to rupture. The damage to the endothelium also disrupts production of endogenous vasodilators and antiplatelet agents such as, nitric oxide and prostacyclin [1, 4].
After disruption of a thin cap of a plaque, some plaques may heal from the fissure, whereas, in others mural thrombi may develop and project into the artery lumen. These thrombi have a high platelet component, which causes the clot to appear white on coronary angioscopic examination. The intraluminal plaques may transiently reduce coronary flow leading to the ischemic condition of the acute coronary syndrome, or they may break up into microemboli, causing focal areas of necrosis and possible arrhythmias [2, 3]. If the intraluminal clot grows rapidly, it may not allow time for the development of collateral flow, predisposing the patient to an AMI. In patients who survive an episode of occlusive thrombus, a natural fibrinolytic reaction normally ensues. Plasminogen is converted to plasmin, which breaks down the fibrinogen and fibrin components of the thrombus restoring distal flow [6-8]. However, usually a high grade lumen stenosis remains, which sets the background for further events. In addition, fibrin debris that remains may contribute to further stenosis or possible total occlusion.
Therefore, it is very important to consider the role of platelets in AMI in order to prevent activation and further aggregation of platelets. The efficacy of acetylsalicylic acid (aspirin), as an antiplatelet agent has been thoroughly investigated, and even today it remains the most widely used and cost-efficient drug in the prevention of platelet aggregation. Ticlopidine, clopidogrel, and prasugrel, potent drugs with demonstrated antithrombotic properties in the prevention of ischemic diseases, have a much higher cost which precludes their widespread general use. Limitations in the use of these drugs have provided the impulse for the search for better antiplatelet drugs. Common antiplatelet agents act by inhibiting different pathways of platelet activation. This may account for the limited ability of these agents to prevent platelet aggregation, since other avenues of activation are left undisturbed. In theory, a drug able to inhibit all of the agonists of platelet activation would necessarily be more effective than the ones that produce only a partial blockade.
PLATELET GP IIb/IIIa RECEPTOR ANTAGONISTS IN AMI
Platelet aggregation is mediated by the GP IIb/IIIa receptor, a member of the integrin family of membrane-bound adhesion molecules. Integrins are defined as subunit receptors composed of an alfa-subunit (GP IIb) and a beta-subunit (GP IIIa) capable of mediated adhesive interactions between cells matrix. It is the chief receptor responsible for platelet aggregation by its ability to bind soluble fibrinogen, which forms bridges between platelets leading ultimately to thrombus formation. Platelet GP IIb/IIIa receptor is distributed widely on platelet surfaces. There are an estimated 40,000 to 80,000 GP IIb/IIIa receptors per platelet, making it the most numerous receptors on the platelet membrane surface [18-20]. However, this receptor remains unable to bind fibrinogen unless the platelet is first stimulated by agonists and undergoes a conformational change [20].
The platelet GP IIb/IIIa receptor is the first integrin to be identified and has served as a model for characterization of other integrins. It is a heterodimer of 2 subunits. The 2 subunits of GP IIb/IIIa are encoded by separate gens on the long arm of chromosome-17. Although each subunit is synthesized separately, the receptor heterodimer must be assembled within the megakaryocyte for either subunit to be expressed [20]. It has been demonstrated by electron microscopy that the receptor is composed of a globular head and 2 flexible tails that are imbebbed in the platelet membrane [21-23]. The GP IIb/IIIa domains responsible for binding adhesive proteins have been identified and in general are characterized by their ability to recognize the peptide sequence RGD. The RGD recognition sequence was originally described for fibronectin but is now known to be present in fibrinogen, von Willebrand factor, vitronectin and thrombospondin [24]. If 2 activated platelets with functional GP IIb/IIIa receptors each bind the same fibrinogen molecule, a fibrinogen bridge is created between platelets. A thrombus forms when this process of aggregation is repeated thousands of times. Since GP IIb/IIIa receptor is unique to platelets and is the final common pathway for platelet aggregation by all agonists, these factors make these receptors a favorable target for therapeutic pharmacologic blockade [21-23].
ABCIXIMAB (Murine Monoclonal Antibodies 7E3)
In 1985, Coller produced a mouse monoclonal antibody against the GP IIb/IIIa integrin receptor [23]. The Coller antibody, termed abciximab or 7E3, has a molecular weight of 47.6 kD and exhibits both a high-affinity and absolute specificity for the GP IIb/IIIa receptor, 2 properties that make 7E3 an attractive agent for the blocking adhesive proteins [24-27]. Abciximab shows a high binding affinity for the GP IIb/IIIa receptor in either the active or inactive state with additional activity against the vitronectin receptor and possibly MAC-1 [28, 29]. The vitronectin receptor plays a role in cell adhesion, migration and proliferation. The vitronectin receptor blockade can prevent smooth muscle cell hyperplasia, and MAC-1 inhibition can prevent stimulated monocyte-induced smooth muscle cell apoptosis. Abciximab has almost no renal excretion, but is cleared rapidly from the plasma. However, it is detectable bound to circulating platelets for at least 21 days [30, 31].
SMALL-MOLECULE AGENTS
Eptifibatide is a cyclic heptapeptide based upon the Lys-Gly-Asp (KGD) amino acid sequence. It has a molecular weight of 0.832 kD and 50% renal excretion [32]. Tirofiban is tyrosine-derivative nonpeptide mimetic with molecular weight of 0.495 kD and 40-70% renal excretion [33]. Lamifiban is also nonpeptide mimetic with a molecular weight of 0.468 kD and 90% renal excretion [34]. Compared with abciximab, these small-molecule agents demonstrate exclusive specificity for GP IIb/IIIa receptor, have less binding affinity, shorter duration of biological effect beyond the administration period and predominantly undergo renal excretion.
Differences in pharmacology have been identified between the agents. Abciximab is derived from the monoclonal antibody 7E3, while the small molecule antagonists are modeled on the alpha-chain (RGD) and the gamma-chain (KQADV) aminoacid recognition sequences in fibrinogen. The inhibition of fibrinogen binding to GP IIb/IIIa receptors is competitive for the small molecule antagonists and non-competitive with abciximab due to its high affinity and slow dissociation rate. Recent findings have made it clear that the different agents interact with separate regions corresponding to, or located close to, the fibrinogen-binding sites on platelet GP IIb/IIIa receptor. Interaction with different sites may explain their differential ability to inhibit platelet-mediated thrombin generation; abciximab is the most potent, tirofiban intermediate and eptifibatide is the weakest [31-33]. In addition to differences in their interaction sites on the GP IIb/IIIa receptor, the small molecules and abciximab differ in their binding specificity. Tirofiban and eptifibatide are highly specific for GP IIb/IIIa, abciximab is less so. Recently, a prospective, randomized trial comparing the platelet effects of abciximab, tirofiban and eptifibatide, demonstrated similar levels of inhibition of platelet aggregation, similar reduction in the platelet-monocyte interaction and similar mean alpha-degranulation, as a measure of antagonist-induced platelet activation [35]. Although, all of these agents reduce ischemic risk to a different level [34, 35], there does seem to be heterogeneity among the drugs with regard to the magnitude and durability of treatment effect, at least in the setting of PCI. Apparent variability among agents in efficacy may be due to pharmacodynamics of receptor binding, with the slow dissociation of abciximab contrasting with the rapid reversibility of other agents. Additionally, the nonspecific blockade by abciximab of both the IIb/IIIa receptor and the vitronectin receptor may theoretically provide an advantage over the specific agents. The suppression of platelet-mediated thrombin generation with dual receptor inhibition is more complete in comparison with inhibition of either receptor alone.
Platelet GP IIb/IIIa antagonist are potent platelet inhibitors, and thus their efficacy is greatest in conditions associated with acute platelet-mediated thrombosis, specifically AMI. Attemps to expand the therapeutic indication of GP IIb/IIIa antagonists to other conditions associated with platelet-mediated thrombosis outside PCI have been less fruitful than expected [31-33].
PLATELET GP IIb/IIIa ANTAGONIST USE IN THE SETTING OF PRIMARY PCI DURING AMI
In the 1990s, platelet GP IIb/IIIa inhibitors were introduced as adjunctive therapy for elective PCI and were soon shown to be effective with primary PCI in the setting of AMI. The greatest magnitude of clinical benefit with these GP IIb/IIIa receptor antagonist agents is achieved in patients undergoing primary PCI, wherein the timing of plaque injury and the role of acute platelet aggregation are precisely defined. Two large randomized international trials demonstrated the unquestionable superiority of stents over balloon angioplasty, mainly because of significantly less incidence of restenosis and reduced need for repeat revascularization [36, 37]. However, stenting activates expression of the GP IIb/IIIa receptor on the platelet surface and predisposes to coronary thrombus, which is partly reflected by the clinical syndrome known as subacute thrombosis [38]. The use of a metal prosthetic device during the procedure may also lead to mural thrombus inside the stent, embolize atherosclerotic material or fragmented thrombus, and predispose to deeper arterial wall trauma and platelet activation because of high pressure inflation. These pathophysiological triggers may be more adequately and effectively controled with the association of platelet GP IIb/IIIa receptor antagonists to stenting. The platelet GP IIb/IIIa receptor antagonists have shown that they can, not only, maintain patency of the recanalized vessel, but also avoid emboli of platelet aggregates to the distal arterial circulation [39]. The addition of platelet GP IIb/IIIa receptor inhibition therapy to PCI resulted in several beneficial effects (Table 1). In an experimental study of stenting and abciximab, it was demonstrated that abciximab treatment reduced platelet thrombus formation area by 89%, but did not prevent the deposition of a discontinuous monolayer of platelet, demonstrating that abciximab blocks platelet to platelet interactions, but not platelet adhesion [40]. The authors of this experimental study proposed that stent insertion leads to a reduction in blood flow inmediately adjacent to the wall, which favors thrombin generation and the development of a fibrin network that can trap the nearby layer of stagnant blood. The most plausible explanation for abciximab’s effect is that it decreases platelet thrombus formation at the sites where the stent struts contact the vessel wall and thus decreases thrombin generation and fibrin formation. There was also a significant smaller platelet thrombus at the site of stent insertion which explains the protective effect of abciximab on the microcirculation [40]. A study designed to assess endothelium-dependent vasomotion after coronary stenting plus abciximab demonstrated that abciximab preserves the coronary blood flow response to acetylcholine after coronary stenting [41]. Acetylcholine-mediated increase in coronary blood flow was impaired after stenting, however when it was associated to abciximab there was a significantly superior response than in the stent alone group [41]. The preservation of microvascular endothelial function may help explain the beneficial clinical effects of GP IIb/IIIa recetor antagonists in patients undergoing primary PCI [41].
Beneficial Effects with the Addition of Platelet GP IIb/IIIa Receptor Inhibitor Therapy to Primary PCI
| 1 | Inhibition of platelet aggregation |
| 2 | Reduced platelet-mediated thrombin generation |
| 3 | Reduced platelet released vasoactive agents |
| 4 | Reduced platelet-mediated clot retraction |
| 5 | Reduced intimal hyperplasia in response to vascular injury |
| 6 | Short- and long-term reduction in ischemic complications |
| 7 | Improved microcirculatory function |
| 8 | Recovery of myocardial function |
| 9 | Reduced long-term mortality |
Randomized Trials Comparing 30-Day Outcomes with Abciximab vs Placebo in Primary PCI for Acute Myocardial Infarction
| Abciximab | Placebo | P | |
|---|---|---|---|
| RAPPORT (42) | (n=241) | (n=242) | |
| Urgent TVR | 1.7% | 6.6% | p<0.006 |
| Composite | 5.8% | 11.2% | p<0.03 |
| ADMIRAL (43) | (n=149) | (n=151) | |
| Urgent TVR | 1.3% | 6.6% | p<0.02 |
| Composite | 6.0% | 14.6% | p<0.01 |
| ISAR-2 (44) | (n=201) | (n=200) | |
| Composite | 5.0% | 10.5% | p<0.04 |
| CADILLAC(45) | (n=1,052) | (n=1,030) | |
| Urgent TVR | 2.5% | 4.4% | p<0.02 |
| Composite | 4.6% | 7.0% | p<0.01 |
| ACE (46) | (n=200) | (n=200) | |
| Reinfarction | 0.5% | 4.5% | p<0.01 |
| Composite | 4.5% | 10.5% | p<0.02 |
TVR=target vessel revascularization. Death, reinfarction, urgent TVR, stroke, and composite end points were evaluated. Only the statistically significant end points are shown. Patients in the ADMIRAL, ISAR-2, and ACE Trials were treated with primary stenting. Only half of CADILLAC patients were treated with primary stenting.
Potential Advantages of Bivalirudin Over Unfractionated Heparin
| 1 | Bivalirudin has more predictable pharmokinetics |
| 2 | It is not inactivated by PF4 |
| 3 | It does not require any cofactor for activity. |
| 4 | It is not inhibited by plasma proteins. |
| 5 | It does not activate platelets. |
| 6 | It is not associated with thrombocytopenia. |
The role of periprocedural GP IIb/IIIa inhibition in the setting of primary PCI during AMI was established by several randomized, placebo-controlled trials enrolling near 4,000 patients [42-46]. Prompt restoration of both epicardial blood flow and myocardial microcirculation are the goals of reperfusion strategies in AMI. As adjunctive therapy for primary PCI, the primary objective of the randomized trials with intravenous GP IIb/IIIa inhibitors was to reduce a 30-day ischemic composite end point (death, MI, urgent revascularization) (Table 2). The most compelling support for platelet GP IIb/IIIa inhibition therapy comes from the abciximab trials. They have demonstrated a clinically important reduction in early ischemic events, sustained beneficial effects at long-term follow-up, and benefits that extends similarly to all interventional devices, lesion complexities and patient acuities. Specifically, there have been 5 large randomized placebo controlled trials evaluating abciximab with UH as adjunctive therapy with primary PCI for AMI [42-46]. All 5 trials have shown improved efficacy with abciximab with a reduction in major adverse cardiac events driven primarily by a reduction in urgent target vessel revascularization (Table 2). A meta-analysis of these 5 trials (RAPPORT, ISAR II, ADMIRAL, CADILLAC and ACE) plus 3 smaller trials by De Luca et al. [47] with a total of 3,949 patients treated with primary PCI found that abciximab reduced 30-day and 6 to 12-month mortality (2.4% vs 3.4%, OR 0.68 [0.47–0.99], P=0.047 and 4.4% vs 6.2%, OR 0.69 [0.52–0.92], P=0.01) and reduced 30 day reinfarction rates (1.0% vs 1.9%, OR 0.56 [0.22–0.94], P=0.03) with no difference in bleeding (4.7% vs 4.1%, P=0.36) [47]. Another meta-analysis of only 3 of these randomized trials (ISAR II, ADMIRAL, CADILLAC) on stent placement in AMI with either adjunctive abciximab or placebo was described [48]. Adjunctive use of abciximab in stent treated patients resulted in an event rate of 12% vs 16.6% without abciximab (OR 0.70; 95% CI 0.54-0.92, p<0.001). Thus, abciximab prevented 44 major events per 1000 patients treated. It was concluded that optimal mechanical reperfusion in AMI is provided by stent placement and GP IIb/IIIa inhibitors use, with a 30% odd reduction in the composite end point. Results from a substudy of the ADMIRAL trial have demonstrated that even in very high risk patients in cardiogenic shock there is a beneficial effect. Death was reduced by 57% with abciximab compared with patients with placebo (9.1% vs 21.4%, NS) [49]. Considering that there were only 25 patients with shock, very small sample size, these results must be interpreted cautiously. Therefore, these results would need further confirmation in a specific trial. Two recent observational, non-randomized studies have shown that treatment with platelet GP IIb/IIIa inhibitor abciximab during angioplasty resulted in improved outcome of patients with shock complicating AMI [50, 51]. Similar results were obtained with eptifibatide in this subgroup of high risk patients. Although, only 25% of the the shock-patients underwent PCI, a retrospective sub-study of the PURSUIT trial has shown that patients treated with eptifibatide seemed to have reduced adjusted odds of 30-day death from shock (OR=0.51, 95% CI 0.28-0.94; p=0.03), suggesting a possible salutary effect of platelet GP II/b/IIIa blockade during shock [52]. Since this finding is derived from a post hoc analysis it should also be verified in specifically designed studies.
Based on these data, abciximab with UH has become a standard adjunctive therapy with primary PCI for AMI and GP IIb/IIIa inhibitors are used in the great majority of patients treated with primary PCI. However, some of the trials were done before the use of stents and the widespread use of thienopyridines. For example, the largest trial, CADILLAC [45], which had a 2x2 randomization to abciximab vs placebo and stenting vs balloon angioplasty, found that the reduction in the 30-day major adverse cardiovascular events rate with abciximab was more pronounced in patients assigned to the balloon angioplasty group (4.3% vs 8.1%, RR 47%, p=0.01) than in those assigned to stenting (4.2% vs 5.3%, RR 21%, NS). Therefore, these findings highlight the beneficial effects of platelet GP IIb/IIIa receptor blockade in AMI patients undergoing primary balloon angioplasty, probably due to the less internal luminal diameter achieved with this procedure compared with stenting. Therefore, this trial found that abciximab reduced the major cardiovascular events in patients treated with ballon angioplasty but not in patients treated with stenting, although it did reduce the frequency of stent thrombosis [45]. Consequently, there has not been universal agreement about the importance of abciximab with primary PCI in the era of clopidogrel and stenting. The 2004 ACC/AHA Guidelines for AMI treatment states that the use of abciximab with primary PCI for AMI is reasonable (Class IIa indication) [53]. This has not been changed in the recent 2007 guidelines update [54].
COMPARISON OF GP IIb/IIIa RECEPTOR ANTAGONISTS WITH BIVALIRUDIN IN AMI: IS IT THE END OF AN ERA?
A combination of antithrombotic and antiplatelet therapies with UH, aspirin, thienopyridine, and GP IIb/IIIa receptor inhibitors has been used to reduce the incidence of ischemic complications during primary PCI in AMI. As Gibson stated, there is a growing body of AMI literature supporting a “union in reperfusion”: the use of pharmacologic agents to quickly open both the artery and the microvasculature and the use of adjunctive interventions to further improve flow and keep arteries open [55]. There are several factors to consider in the safety profile of the platelet GP IIb/IIIa receptor inhibitors. Platelet inhibition of >80% is required for maximum efficacy [56]. Failure to achieve these high levels of inhibition is associated with lack of protection from acute events. Moderate levels of platelet inhibition not only lack efficacy but are associated with a paradoxical increase in ischemic complications due to the unmasking antagonist-induced prothrombotic and proinflammatory effects [56-59]. During an abciximab infusion, more than 25% of patients are less than 80% inhibited increasing the possibility of thrombotic, counterregulatory events. The reduction of bleeding complications in the PCI plus GP IIb/IIIa antagonists trials demonstrates that reduction of concomitant heparin doses, as well as early vascular sheath removal ameliorate excess hemorrhagic risk in this setting. For patients who develop refractory or life-threatening bleeding, the anti-platelet effect of abciximab may be reversed by discontinuation of drug infusion and by platelet transfusion after the 10 to 30 min required for clearence of circulating drug. Platelet transfusions should rarely be necessary with rapidly reversible agents such as eptifibatide and tirofiban, and in fact, might not be expected to be effective during the 2 h required for elimination of these agents from the circulating plasma phase [60]. Thrombocytopenia occurs infrequently with GP IIb/IIIa inhibition but may be precipitous and profound (platelet count of <50,000 mm3) [60]. Therefore, platelet count should be measured early (within the first 2 to 4 h) after administering these agents and followed for the duration of therapy. Pseudothrombocytopenia is the cause of more than one third (36%) of low platelet counts in patients undergoing PCI who are treated with abciximab. Pseudothrombocytopenia is a benign laboratory condition that does not increase the risk of bleeding, stroke, transfusion requierements or the need for repeat revascularization. Besides, it does not require specific therapy. Therefore, it is important to recognize this condition so that the beneficial effects of abciximab are not lost by premature termination of therapy [60]. In this regard, it is important to note that due to its murine origin, abciximab elicits an immune response in the form of IgG antibody production, termed the human antichimeric antibody (HACA), in 7% of patients within the first month after initiation of therapy. This does not occur with the small molecule antagonists. Abciximab readministration in this situation is safe and does not result in an allergic response or any increase in adverse events, although, increased levels of thrombocytopenia are seen. The presence or absence of a positive HACA titer was not predictive of a lack of clinical effectiveness, development of thrombocytopenia, or other sequelae in patients undergoing readministration [60]. Thus, overall side effects are similar with the different agents and usually not a major clinical problem.
Although the use of more potent antithrombin and antiplatelet agents during PCI has reduced the rate of peri and post-procedural ischemic complications, in parallel, the rate of bleedings has increased [61]. Of importance, numerous studies have reported an association between bleeding after PCI and an increase in short- and long-term morbidity and mortality [61-63]. Bivalirudin, a direct thrombin inhibitor, was developed as an antithrombin agent for patients undergoing PCI with the hypothesis that it would reduce bleeding complications without compromising the rate of ischemic events compared with UH plus GP IIb/IIIa inhibitors. Consistently, trials performed in various clinical settings, from stable angina to acute coronary syndrome, have demonstrated that the strategy of bivalirudin with provisional GP IIb/IIIa inhibitors was superior when compared with UH with systematic GP IIb/IIIa inhibitors in regard to reduction in bleeding complications without any increase in the rate of ischemic events [10-12]. The recent Harmonizing Outcomes with RevascularIZatiON and stents in ST Elevation Myocardial Infarction (HORIZON STEMI) trial expanded these findings to primary PCI in AMI [13].
Bivalirudin is a semi-synthetic 20 amino-acid polypeptide derived from native hirudin [13]. This compound binds specific for unbound and bound thrombin at a 1:1 ratio. It has potential advantages over UH (Table 3) [13]. The HORIZONS Trial evaluated bivalirudin in AMI patients treated with primary PCI [13]. Bivalirudin, compared with UH plus platelet GP IIb/IIIa inhibitors, resulted in less major bleeding (4.9% vs 8.3%, P<0.001) (Fig.1A), and similar rate of major adverse cardiovascular events at 30 days (5.4% vs 5.5%, P=0.95) (Fig.2A). The primary endpoint, a composite of major adverse cardiovascular events or major bleeding (net adverse clinical events), was significantly less with bivalirudin (9.2% vs 12.1%, P=0.005) (Fig.3). Furthermore, 30-day mortality was significantly less with bivalirudin (2.1% vs 3.1%, P=0.047), and there was less thrombocytopenia (0.5% vs 1.1%, P=0.02). The frequency of stent thrombosis in the first 24 h after PCI was greater in the bivalirudin group (1.3% vs 0.3%, P <0.001). However, at 30 days, there were no significant differences between groups (2.5% vs 1.9%, P=0.30). The HORIZONS trial [13] is the first to show a significant reduction in mortality with any adjunctive therapy used with primary PCI in AMI patients. However, this result could have occurred by chance and should be considered with caution, because mortality was not a primary or secondary endpoint. In addition, the study was not powered to detect differences in mortality between both groups. Nevertheless, there are certain factors that could explain this mortality difference. Major bleeding complications in patients with acute coronary syndromes treated with PCI have been shown to be strong predictors of subsequent stent thrombosis, other ischemic events and death [64, 65]. Moreover, major bleeding has been shown to be the strongest predictor of 30-day mortality in PCI-treated patients with acute coronary syndromes, even stronger than periprocedural AMI [64, 65]. Major bleeding can contribute to mortality because of mortality from the bleeding event itself, the need for transfusions, and the need for discontinuation of antiplatelet and anticoagulant therapy which may predispose to stent thrombosis and other ischemic events. Blood transfusions are associated to significantly increased morbidity and mortality, increased infections, and increased hospital stay and costs [66, 67]. Thrombocytopenia is also associated with worse outcomes, and the reduction in thrombocytopenia with bivalirudin may also contribute to lower mortality.
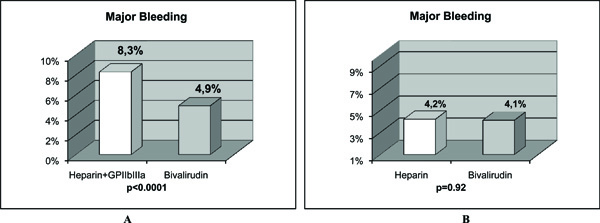
The results of major bleeding in 2 studies with AMI patients treated with primary PCI are shown. The comparison of bivalirudin to heparin plus GP IIb/IIIa inhibitors in the HORIZONS trial (Fig. 1A), and, to heparin alone in the study of Bonello L et al (72), (Fig, 1B) is depicted. When bivalirudin is compared with heparin, there is only a significant difference in major bleeding in AMI patients undergoing PCI only when GP IIb/IIIa inhibitors are systematically added to unfractionated heparin, but not when bivalirudin is compared to heparin alone without the use of GP IIb/IIIa inhibitors.
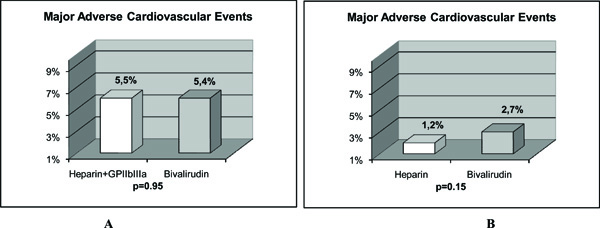
The results of major adverse cardiovascular events in 2 studies with AMI patients treated with primary PCI are shown. The comparison of bivalirudin to heparin plus GP IIb/IIIa inhibitors in the HORIZONS trial (2A) (13), and, to heparin alone in the study of Bonello L et al (2B) is depicted. There was no significant difference in major adverse cardiovascular events in AMI patients undergoing PCI when bivalirudin was compared with unfractionated heparin with or without the use of GP IIb/IIIa inhibitors.
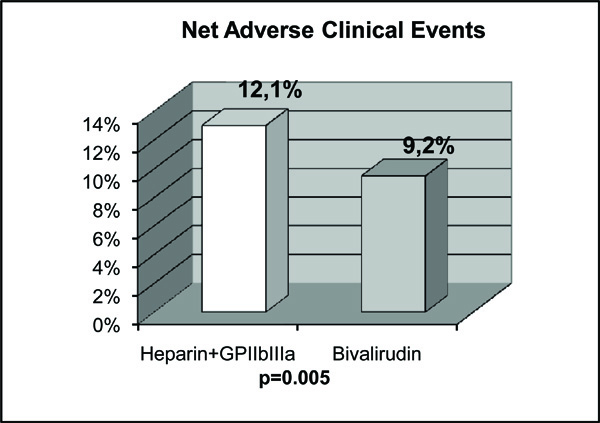
The results of net adverse clinical events in the HORIZONS trial (13) are shown. In the comparison of bivalirudin with heparin plus GP IIb/IIIa inhibitors, there was a significant difference in the net adverse clinical events in AMI patients undergoing PCI. That is, the statistical significance was obtained only when major bleeding was added to conventional major adverse cardiovascular events.
The results of HORIZONS trial [13] make a strong argument for the use of bivalirudin rather than UH plus GP IIb/IIIa inhibitors for the great majority of AMI patients treated with primary PCI. Does this mark the end of an “old era” (GP IIb/IIIa inhibitors) and the beginning of a “new era” (bivalirudin)? Probably this is the beginning of a more rationale use of GP IIb/IIIa inhibitors, since certain patients may still benefit by their use. UH plus GP IIb/IIIa inhibitors still have potential advantages in patients with high clinical risk but low bleeding risk. Patients with cardiogenic shock may do better with UH plus GP IIb/IIIa inhibitors rather than bivalirudin alone. Another group of patients who may benefit from GP IIb/IIIa inhibitors are patients with angiographically documented large or giant thrombus, patients with stent thrombosis, and patients who develop refractory no-reflow phenomenon following PCI.
There are several limitations of the trial [13] design and results that merit careful consideration. First, the limitation of an open-label design requires emphasis, as it creates potential for bias. This study design weakens the conclusiveness of any analysis of end points, such as bleeding and ischemic events. Second, the effect of the administration of another antithrombin agent (UH) in approximately 65% of the patients in the bivalirudin group shortly before PCI warrants consideration. Therefore, bivalirudin was tested as monotherapy in only 615 patients. In this group, major cardiovascular events occurred in 7.2% of the patients, as compared with 5.2% of the patients who received UH plus GP IIb/IIIa inhibitor (relative risk, 1.39; 95% CI, 0.85 to 2.28, P<0.1). The administration of UH before enrollment to the majority of patients in the study mitigates the strength of the results with respect to clinical practice in which bivalirudin might be used as monotherapy. Third, while the study is relatively large, it is underpowered to evaluate infrequent safety endpoints, such as stent thrombosis. Fourth, Abciximab was used in only 52% of patients in the UH plus platelet GP IIb/IIIa inhibitor group while eptifibatide and tirofiban, which have not been extensively evaluated in AMI patients, were used in 46% of patients. Also, the comparison of bivalirudin with provisional use of GP IIb/IIIa inhibitors vs mandatory use of GP IIb/IIIa inhibitors in association with UH in the HORIZONS trial [13] was subject to criticism. In fact, studies have shown that GP IIb/IIIa inhibitor use is associated with a reduction in ischemic recurrence at the cost of increased bleeding complications. Of importance is that their benefit is confined to high-risk patients and, therefore, revealed to be useless and even harmful in low-risk populations [48, 68]. Thus, the use of systematic GP IIb/IIIa inhibitors in the UH arm may be questionable. It may be questionable even in AMI patients, especially when, in the real world practice, only 20–58% of patients receive them despite their potential advantages and according to guidelines [69-71, 54]. In addition, the benefit observed with bivalirudin was achieved because of the major bleeding complications with UH plus GP IIb/IIIa inhibitors. Therefore, what would happen if we diminish bleeding complications by eliminating GP IIb/IIIa inhibitors from the equation? There are some studies that compared bivalirudin with UH alone.
COMPARISON OF UH WITH BIVALIRUDIN IN AMI: THE BEGINNING OF A NEW ERA?
There is no randomized trial comparing bivalirudin with UH alone in AMI patients undergoing primary PCI. Therefore, whether or not bivalirudin continues to show similar efficacy and reduced bleeding when compared with UH without GP IIb/IIIa inhibitor infusion in AMI patients treated with primary PCI is an unanswered question by large, randomized trials. However, there are some observational studies in this setting that can be analyzed.
Bonello L et al. [72] evaluated the safety and efficacy of bivalirudin vs UH when used without GP IIb/IIIa inhibitors during primary PCI in AMI patients. The rate of major bleeding was identical in the 2 groups (4.1% vs 4.2%, P=0.92) (Fig.1B). Although there was a trend toward less hematoma in the bivalirudin group, it did not reach significance (3.4% vs 6.7%, P=0.09). The rate of major hematoma was identical between the 2 groups as well (0.5% vs 0.6%, P=1). There was no difference in the rates of death (1.1% vs 0.9%, P=1), or major adverse cardiovascular events (2.7% vs 1.2%, P=0.15) (Fig.2B)in the bivalirudin and UH groups, respectively. No stent thrombosis occurred during in-hospital stay. Therefore, the results of this study [72] suggest that in primary PCI for AMI, bivalirudin vs UH when used without GP IIb/IIIa inhibitors exhibits similar results in terms of ischemic and bleeding complications. This could be of major interest in patients with high risk of bleedings or low risk of ischemic events, which do not receive GP IIb/IIIa inhibitors during PCI for AMI. A better ischemic outcome in patients with bivalirudin would have been expected due to the lack of GP IIb/IIIa inhibitors in the UH group. However, another study in AMI patients comparing bivalirudin with UH, also reported identical outcomes between the 2 drugs with GP IIb/IIIa inhibitors used only as ‘bailout’ in both groups [73].
Bittl JA et al. [74] compared bivalirudin with UH in patients undergoing PCI. Although, their results were not obtained from AMI patients, bivalirudin did reduce the incidence of bleeding complications as compared with UH in this trial. However, since it was performed more than 15 years ago, the exclusive use of balloon angioplasty, the lack of treatment with clopidogrel, the high dose of UH administered, and the outdated regimen of bivalirudin make the trial results largely irrelevant to current interventional practice [74]. Nevertheles, Kastrati A et al. [75] observed similar results in a contemporary PCI setting. They compared bivalirudin with UH in stable or unstable angina patients who did not have elevated levels of biomarkers, and who were undergoing PCI after having received pretreatment with 600 mg of clopidogrel at least 2 h before the procedure. The main finding was that bivalirudin did not provide a net clinical benefit. It did not significantly reduce the incidence of the quadruple end point (death, myocardial infarction, urgent target-vessel revascularization, or major bleeding) compared with UH at 30 days, although it did significantly reduce the incidence of bleeding. Therefore, in the setting of PCI and in the absence of GP IIb/IIIa inhibitors, bivalirudin did not offer any beneficial effect in the incidence of the composite end points. The benefit observed in the incidence of bleeding in stable or unstable angina patients was not observed in AMI patients. As we can see in Fig. (1), when bivalirudin is compared with UH, there is only a significant difference in major bleeding in AMI patients undergoing PCI only when GP IIb/IIIa inhibitors are systematically added to UH (Fig. 1A), but not when bivalirudin is compared with UH alone without the use of GP IIb/IIIa inhibitors (Fig. 1B). In addition, we can see in Fig. ( 2), that when bivalirudin was compared with UH plus GP IIb/IIIa inhibitors in the HORIZONS trial [13] (Fig. 2A), and, with UH alone in the study of Bonello L et al. [72] (Fig. 2B), there was no significant difference in major adverse cardiovascular events in AMI patients undergoing PCI. Moreover, as shown in Fig. (3)in the comparison of bivalirudin with UH plus GP IIb/IIIa inhibitors, there was a significant difference in the net adverse clinical events in AMI patients undergoing PCI in the HORIZONS trial only when major bleeding was added to the conventional major adverse cardiovascular events. Therefore, it is major bleeding in the UH plus GP IIb/IIIa inhibitors group which plays a major role in favoring a better outcome and mortality rate in the bivalirudin group. Hence, is it the beginning of a new era with bivalirudin or is it a welcome back to an old friend UH? Although, these questions can only be answered with certainty with large, double blind, randomized trials in AMI patients undergoing PCI, the results of the studies available until now exert some doubts in the future widespread use of bivalirudin. For now, in the real world practice, one would probably choose a well known cheaper drug that has already passed the test of time, UH.
CONCLUSION
Intravenous platelet GP IIb/IIIa receptor antagonists represent a significant advance in the practice of interventional cardiology and have proven to be an important, clinically effective adjunct therapy during primary PCI in AMI patients. Adding platelet GP IIb/IIIa receptor inhibition therapy to coronary stenting has demonstrated to have short and long-term reduction in ischemic complications, improved microcirculatory function and recovery of myocardial function. Although their utilization has reduced the rate of peri- and post-procedural ischemic complications, in parallel, the rate of bleeding has increased. In addition, suboptimal levels of GP IIb/IIIa receptor antagonists are not only nonprotective and prothrombotic, but they also promote inflammation. In the light of recent data, there will be a more rational use of platelet GP IIb/IIIa inhibitors. They still have potential advantages in patients with high clinical risk but low bleeding risk. Patients with cardiogenic shock may do better with UH plus IIb/IIIa inhibitors rather than bivalirudin alone. Another group of patients who may benefit from GP IIb/IIIa inhibitors are those with angiographically documented large or giant thrombus, patients with stent thrombosis, and patients who develop refractory no-reflow phenomenon following PCI.
Bivalirudin, a direct thrombin inhibitor, was developed as an antithrombin agent for patients undergoing PCI with the hypothesis that it would reduce bleeding complications without compromising the rate of ischemic events compared with UH plus GP IIb/IIIa inhibitors. It was demonstrated that the strategy of bivalirudin with provisional GP IIb/IIIa inhibitors was superior when compared with UH plus systematic GP IIb/IIIa inhibitors in regard to reduction in bleeding complications without any increase in the rate of ischemic events in AMI patients during primary PCI. However, in the setting of PCI and in the absence of GP IIb/IIIa inhibitors, bivalirudin did not offer any beneficial effect in the incidence of the composite end points when compared with UH. The benefit observed in the incidence of bleeding in stable or unstable angina patients was not observed in AMI patients. Therefore, the results of the studies available until now set some doubts in the future widespread use of bivalirudin. For now, in real world practice, one would probably choose a well known cheaper drug that has already passed the test of time, UH. It is rewarding to see that there is so much work already done in this field, but there is still a long way to go with further investigations to shed more light on yet unanswered questions.


