All published articles of this journal are available on ScienceDirect.
Effect of Intermittent Versus Continuous Parathyroid Hormone in the Cardiovascular System of Rats
Abstract
Objective:
PTH increases ionic calcium concentration in the serum, acting primarily on bone and kidney cells through the type 1 PTH receptor. Interestingly, PTH stimulates bone formation when administrated intermittently but causes severe bone loss with continuous administration. Daily injections of PTH are used as the most promising anabolic agent in the treatment of severe osteoporosis. Elevated PTH is reported an independent risk factor for left ventricle hypertrophy.
Design:
in rats we investigated the effect of intermittent and continuous administration of PTH on blood pressure, heart rate and development of cardiac hypertrophy and fibrosis.
Results:
We did not find PTH to induce heart hypertrophy. In contrast, continuous administration of PTH the mRNA level of a hypertrophic marker gene, atrial natriuretic peptide. When comparing the effect of continuously versus injected PTH collagen 1 mRNA was significantly higher in continuously treated animals.
Conclusion:
our data demonstrated a decrease in heart rate upon continuous administration of PTH in rats. No changes in blood pressure were observed. Moreover, neither intermittent nor continuous administration of PTH induced ventricular hypertrophy. But continuous PTH induced a marker of collagen 1. Thus, these data did not reveal any negative effects of the injection of PTH on the cardiovascular system.
1. INTRODUCTION
Parathyroid hormone (PTH) is a peptide hormone secreted by the parathyroid glands. PTH is the most important endocrine regulator of short-term calcium homeostasis. Concentration of ionic calcium (Ca2+) in extracellular fluid is sensed by the calcium-sensing receptor residing on the surface of the cell membrane of the parathyroid cells. A decrease in Ca2+ induces a release of PTH, whereas an increase in Ca2+ level has an inhibitory effect on the PTH secretion through the calcium-sensing receptor [1-3]. In turn, PTH increases calcium concentration primarily by acting on bone and kidney cells through the type 1 PTH receptor (PTH1R), causing the linning cells of the bones to release calcium into the blood and enhancing the amount of calcium reabsorped along the nephron.
PTH (1-34) is a promising anabolic agent to date in the treatment of osteoporosis [4]. This is due to direct stimulation of bone formation. PTH injections improve bone qualities, increase bone mass and prevent osteoporotic fractures. However, the effects of PTH are paradoxical as PTH stimulates bone formation when injected once daily but causes severe bone loss when continuously infused or secreted by the parathyroid glands as in hyperparathyroidism [5].
In addition to the major PTH target organs, the kidney and bone, PTH also has effects on other tissues, including heart and blood vessels [6]. PTH1R is widely expressed throughout the cardiovascular system, including heart [7]. Moreover, mice lacking the PTH1R die prenatally with massive cardiac necrosis, indicating that PTH1R is required for a normal development of the heart [8].
Clinical data indicate that elevated PTH contributes to the progression of several cardiovascular diseases. Patients with end-stage renal diseases, which exhibit elevated plasma concentrations of PTH, develop severe left ventricular hypertrophy. Furthermore, cardiovascular diseases are the leading cause of mortality in end-stage renal disease patients [9, 10]. Moreover, parathyroidectomy in patients with extremely high serum PTH levels and accelerated left ventricular mass results in a marked reduction of both PTH levels and left ventricular mass [11, 12]. PTH is also an independent predictor of left ventricular mass by high estimated with M-mode echocardiography in males older than 59 years and females younger than 60 years [13]. This effect is only seen when PTH is substantially elevated.
These clinical data are supported by in vitro studies that demonstrate a direct hypertrophic effect of PTH on isolated adult ventricular cardiomyocytes [14]. Moreover, PTH increases beating frequency and Ca2+ influx of cardiomyocytes [15, 16].
In this study, we aimed to investigate the effect of intermittent and continuous administration of PTH on blood pressure, heart rate and development of heart hypertrophy and fibrosis in rats.
2. METHODS
2.1. Animal Model
Male Wistar rats 200–220 g were anesthetized by subcutaneous injection of Hypnorm/ Dormicum for a chronic catheterization procedure. Through the right internal carotid artery, one catheter (medical-grade Tygon tubing) was placed with its tip in the ascending aorta. The catheter was subsequently filled with a solution containing 0.5 ml glucose (500 g/l), heparin (100 IU) and streptokinase (5000 IU) and plugged with a nylon pin. Each catheter was immediately externalized through the neck skin region and secured by a polyester felt disk placed in the subcutis [17].
Following the catheter implantation, the rats were housed individually (12 h light/ 12 h dark) with unlimited amounts of standard rat chow and tap water. Post-operatively, the rats were allowed to recover for at least 5 days in order to obtain their pre-operative weight.
Before entering the test program both test and control animals base-line blood pressure (BP) were measured using pressure transducers (Baxter Corp) connected to the right arterial catheter. Hart rate (HR) was derived from the arterial pressure signal. Tracings of the variables were displayed on a Watanabe linear recorder (Watanabe Instruments Corp., Japan [17]).
Animals were conscious and unrestrained throughout measurement of BP and HR. After baseline hæmodynamics were measured, PTH (1-34) was administered either continuous or pulsative during 2 weeks. Pulsative administrations were performed once daily (30 µg/kg/day) or twice daily (15 µg/kg/day) as a subcutaneous bolus injections of PTH. Continuous administrations were performed using an osmotic minipump (Alza Corp, 2002 12 µl/d was placed subcutaneously delivering PTH (6 µg/day) or placebo (acetic acid (vehicle)) at a constant rate of 12 µl/d throughout the study period.
At the end of the study period the BP and HR of the animals were measured and recorded. Subsequently, all animals were sacrificed and blood samples were drawn from aorta and analysed on a Ciba Corning 634 Ca++ /pH Analyzer® , ion-selective electrodes to estimate the serum Ca2+ levels (ionic calcium) [18] . Hearts were removed and weighed (total weight, left and right ventricle weight) and snap frozen in liquate nitrogen. The length of the femur was measured before being snap frozen in liquid nitrogen.
All experiments conform with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health NIH publication no. 85-23, revised 1996.
2.2. Quantification with Real-Time PCR
Total RNA was isolated from 10-30 mg of heart tissue (left ventricle) with RNeasy Fibrous Tissue Kit (Qiagen) after homogenization with a Mixer Mill 300MM (Retsch, Haan, Germany). RNA purity and concentration were determined by A260 and A280 measurement. First-strand cDNA was synthesised from 1μg of total RNA using Omniscript RT kit (Qiagen).
Quantitative real-time PCR analyses were performed with the Rotor-Gene 3000 (Corbett Research, Mortlake, Australia) and Quantitect SYBR Green PCR Kit (Qiagen) as described previously [19]. The primers were:
GAPDH, forward: 5'- GTC GGT GTG AAC GGA TTT G- 3'
GAPDH, reverse: 5'- CTT CCC GTG GGT AGA GTC AT -3'
Collagen-I, forward: 5'- AGC TTC ACC CTT AGC ACC AG- 3'
Collagen-I, reverse: 5'- GTG GTA ACG ATG GTG CTG TC- 3'
Collagen-III, forward: 5'- ACC AGG GTC GCC ATT TCT- 3'
Collagen-III, reverse: 5'- GCC TTC TAC ACC TGC TCC TG- 3'
Fibronectin, forward: 5'- GTT TTG ACA ACG GGA AGC AT- 3'
Fibronectin, reverse: 5'- TCT TCA GGT TCA GGC TTG CT- 3'
ANP, forward: 5’- CCG GTA CCG AAG ATA ACA G-3’
ANP, reverse: 5’- CTC CAG GAG GGT ATT CAC C-3’
The specificity of each set of primers was ensured by 1.4% agarose gel analysis and DNA sequence analysis (GATC Biotech, Konstanz, Germany). All mRNAs were quantified in duplicates and the results were normalized to the content of GAPDH within the same sample.
2.3. Calculations and Statistics
MAP was estimated, in mmHg, as diastolic pressure plus (systolic minus diastolic pressure) divided by 3. All data were analyzed with GraphPad Prism using two-tailed unpaired t-test. P < 0.05 was considered significant. n= number of animals
3. RESULTS
3.1. Continuous Administration of PTH
A continuous infusion of PTH or vehicle was given for a period of 14 days. By end of the treatment, PTH animals were compared to vehicle treated animals (controls). PTH in the mini pumps was released as reflected in the serum ionized calcium level, as serum Ca2+ was significantly higher in the PTH treated animals compared with controls (p=0.02) (Fig. 1a). No significant difference was found in body weight, lung weight, tibia length, or BP (data not shown). Moreover, no visible signs of heart hypertrophy were observed, as measured by the left ventricle mass (Fig. 1b). However, mRNA level of a hypertrophic marker gene, atrial natriuretic peptide (ANP), was significantly increased in the PTH treated animals (p=0.04) (Fig. 2a). No changes in mRNA levels of fibrotic gene markers, collagen 1, collagen 3 and fibronectin were observed (Figs. 2b-d). In the end of the experiment, heart rate tended to be lower in the PTH treated animals (no significance, p=0.08) (Fig. 3a). No difference was observed before the experiment (Fig. 3b). Excluding 2 clear outliers in the control group, the change in the heart rate from baseline to end of the experiment (delta heart rate) was significantly lower in the PTH animals (p=0.01) (Fig. 3c).
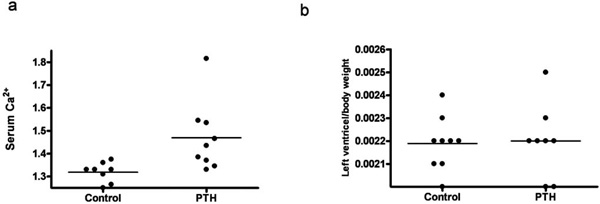
Effect of continuous administration of PTH on: (a) Serum Ca2+ level. (b) Left ventricle mass. Each point represents a value from an individual rat.
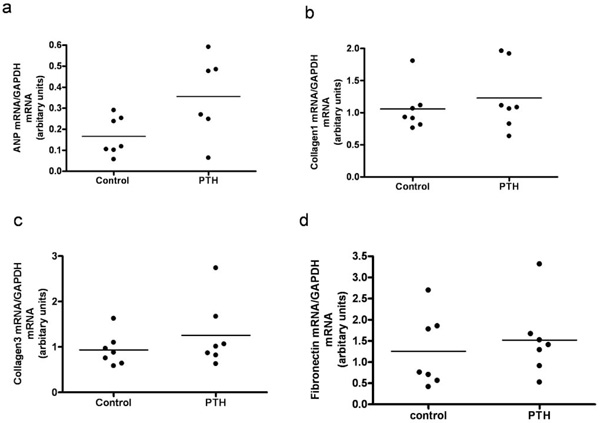
Left ventricular expression of mRNAs for atrial natriuretic peptide (ANP) (a), Collagen1 (b), Collagen3 (c) and Fibronectin (d) in rats treated with continuous administration of PTH. Each point represents a value from an individual rat.
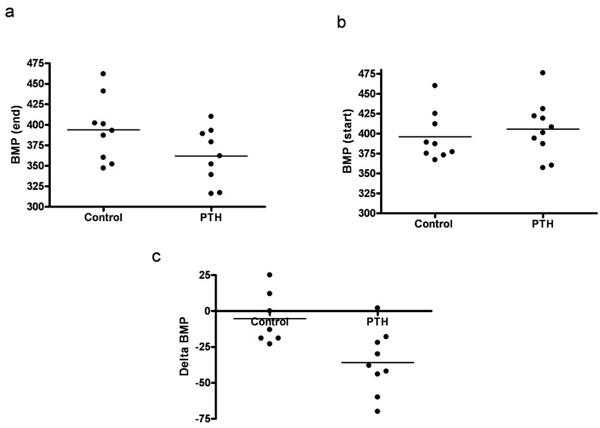
Effect of continuous administration of PTH on heart rate (BMP). (a) Heart rate by the end of the experiment. (b) Heart rate in the beginning of the experiment. (c) Delta heart rate (excluding 2 outliners from the control group). Each point represents a value from an individual rat.
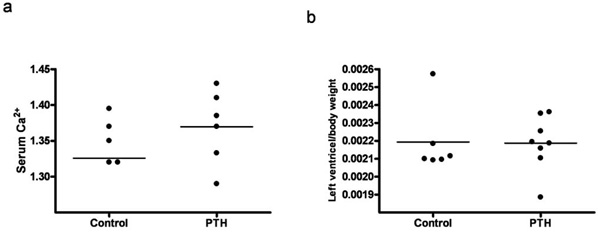
Effect of intermittent administration of PTH on: (a) Serum Ca2+ level. (b) Left ventricle mass. Each point represents a value from an individual rat.
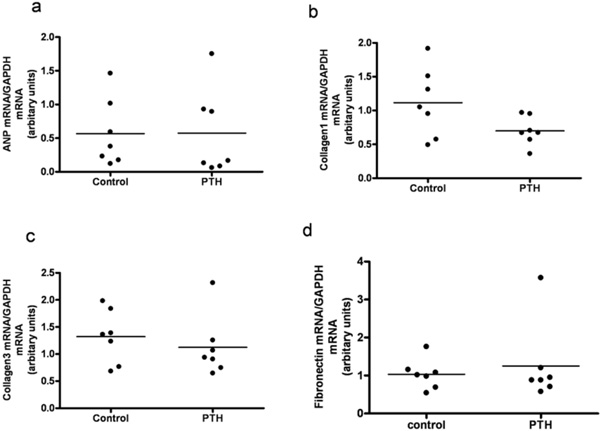
Left ventricular expression of mRNAs for atrial natriuretic peptide (ANP) (a), Collagen1 (b), Collagen3 (c) and Fibronectin (d) in rats treated with intermittent administration of PTH. Each point represents a value from an individual rat.
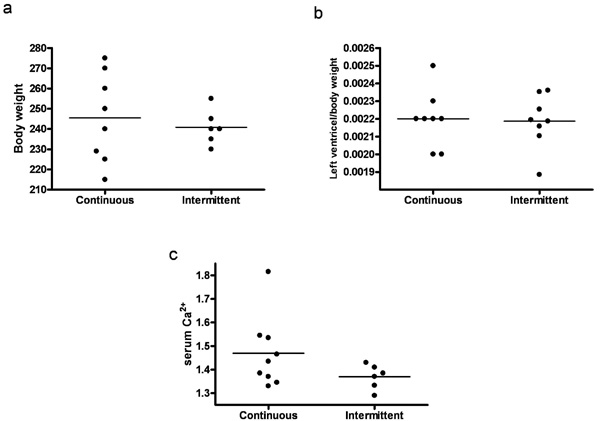
Effect of continuous versus intermittent administration of PTH on: (a) Body weight. (b) Left ventricle mass. (c) Serum Ca2+ level. Each point represents a value from an individual rat.
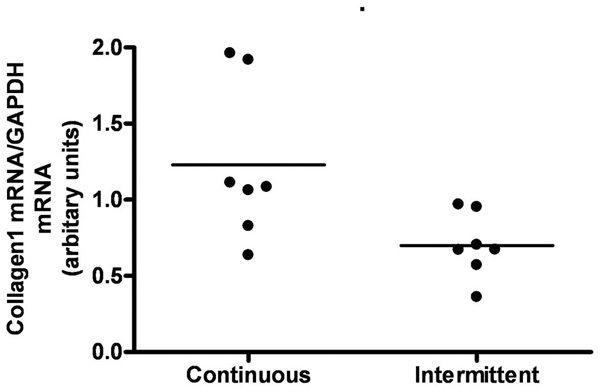
Effect of continuous versus intermittent administration of PTH on left ventricular expression of mRNA for Collagen1. Each point represents a value from an individual rat.
3.2. Intermittent Administration of PTH
Animals injected with PTH did not differ in body weight, lung weight or tibia length from animals injected with vehicle (data not shown). Serum Ca2+ tended to be higher in the PTH injected animals, however not significantly (Fig. 4a). No changes in the left ventricle mass or ANP, collagen 3 and fibronectin mRNA levels were observed in the PTH treated group (Figs 4b, 5a, 5c, and 5d). Expression of collagen1 mRNA tended to be lower in the PTH injected animals, however not significantly (Fig. 5b).
3.3. Continuous Versus Intermittent Administration of PTH
We compared animals treated with continuous and intermittent administration of PTH. No significant difference in body weight or left ventricle mass was observed (Figs 6a and 6b). Serum calcium tended to be higher in animals treated with continuous administration of PTH, however not significantly (Fig. 6c). Moreover, expression of collagen 1 mRNA was significantly higher in continuously treated animals (p=0.03) (Fig. 7).
4. DISCUSSION
PTH is the most important regulator of calcium homeostasis and a key factor in the control of bone modelling. Interestingly, PTH has opposite effects on bone, depending upon the mode of application [5]. Brief daily exposuresresult in anabolic effects, while continuously administration at a sustained level has catabolic effects. Daily injections of PTH are used as a novel and promising anabolic treatment for osteoporosis [4].
Although PTH acts primarely on bone and kidney cells, it is well known that cardiovascular cells are target cells for PTH [20]. PTH increases calcium influx in cardiomyocytes but decreases calcium influx in smooth muscle cells [20]. Moreover, PTH exerts a direct hypertrophic effect on cardiomyocytes and increases their beating frequency [14-16].
In this study, we examined effects of PTH on BP, heart rate and left ventricle mass in rats.
The effect of PTH on BP is complex. It causes acute vasodilation and lowers BP in rats [21]. However, infusion of human PTH (1-34) in healthy volunteers causes an acute modest increase in BP [22]. Recently, Zaruba et al. observed a decrease in BP after PTH injections in mice [23]. This study did not include a control (vehicle treated) group, and can therefore not exclude that the decrease in BP occurred spontaneously over time and is not an effect of PTH.
It is important to keep in mind the possibility of species-related differences and of different short-term versus long-term BP effects of PTH. Although PTH causes acute decrease in BP in rats, it has been proposed that this acute BP-lowering effect might be suppressed in the long run by an elevation of the BP that results from cellular calcium loading [24]. Accordingly, continuous administration of PTH did not have any effect on BP in our study.
PTH has previously been demonstrated to induce an increase in heart rate both in cultured cardiomyocytes [15, 16] and in isolated perfused hearts [25]. Moreover, intermittent administration of PTH in rats has also been reported to produce an increase in heart rate [26]. However, in the present study, continuous PTH administration led to a slowing of the heart rate. Interestingly, it has previously been demonstrated that an overexpression of the PTH1R in rats decreases heart rate [27]. The mechanisms underlying this negative chronotropic effect are not clear.
Our data, together with previous studies, suggest that intermittent administration of PTH leads to an acute increase in heart rate, while continuous administration of PTH decreases heart rate.
PTH has previously been demonstrated to directly induce hypertrophy of isolated rat cardiomyocytes [14]. In addition, 70% of patients with end-stage renal disease, which exhibit elevated plasma PTH levels, suffer from severe left ventricular hypertrophy [10, 28]. Therefore, we investigated the effect of both intermittent and continuous administration of PTH on left ventricle mass. Neither treatment led to development of hypertrophy in our study, measured by left ventricle mass. However, expression of a hypertrophic marker gene, atrial natriuretic peptide (ANP), was significantly increased in animals treated with continuous administration of PTH compared with controls, suggesting that hypertrophic process appeared to begin. The lack of visible signs of a hypertrophic heart (increase in left ventricle mass) could be caused by a too short exposure compared with the human observations.
We also investigated mRNA expression of fibrotic gene markers, collagen 1, collagen 3 and fibronectin. Neither treatment led to significant changes in expression of the fibrotic gene markers. However, level of collagen 1 mRNA tended to be lower in animals treated with intermittent administration of PTH.
In summary, our data demonstrate, to our knowledge for the first time, a decrease in heart rate upon continuous administration of PTH in rats. No changes in BP were observed. Moreover, neither intermittent nor continuous administration of PTH induced ventricular hypertrophy or a fibrotic process in the timeframe of this study.
ACKNOWLEDGEMENTS
This work was supported by grants from The Danish National Research Foundation and The John and Birthe Meyer Foundation to J.T.H.; and Copenhagen University to S.S.


