All published articles of this journal are available on ScienceDirect.
High Sensitivity C-reactive Protein in Patients with Coronary Artery in-stent Restenosis: A Case-control Study
Abstract
Background:
Inflammation plays a pivotal role in the pathogenesis of In-Stent Restenosis (ISR). High sensitivity C-reactive protein (hsCRP) is positively associated with major cardiovascular events.
Aim:
We aimed to investigate the hsCRP inflammatory response to Percutaneous Coronary Intervention (PCI) in Coronary Artery Disease (CAD) patients with coronary ISR vs. patients without ISR.
Methods:
This case-control study included 80 CAD patients previously treated with drug-eluting stent (DES) implantation. Patients had Coronary Angiography (CAG) because of chest pain or equivalent symptoms and were subdivided into 2 groups. Group A (n=40) included CAD patients with ISR. Group B (n=40) included age and gender-matched controls with CAD but without ISR. Serum hsCRP levels were obtained before PCI (baseline) and 8, 16, 24 h post-PCI.
Results:
At baseline (before intervention/CAG), the hsCRP level was increased in the ISR group compared with the No-ISR group (p=0.007). There were 36 (90%) patients in the ISR group who had a high hsCRP (>3 mg/L) compared with 25 (62.5%) patients in the No-ISR group. Also, there was a significant relationship between high hsCRP and the ISR. Patients with ISR had higher frequencies and percentages of elevated CRP than the no-ISR control group. This difference was maintained for all measurements, baseline, after 8, 16, and 24 h (p<0.05). Repeated measures analysis of variance (ANOVA) in the ISR group revealed that mean hsCRP differed significantly between serial measurements (p<0.001). In contrast, in the control group, the mean hsCRP did not differ significantly between the serial measurements (p=0.65).
Most of our patients (n=66, 82.5%) had 1-vessel CAD disease, and the left anterior descending (LAD) coronary artery was significantly affected in 46 patients (57.5%). Management of restenosis was accomplished mainly by stenting by DES in 29 patients (72.5%).
Conclusion:
Patients with ISR had substantially higher pre- and post-PCI hsCRP levels than the no-ISR controls. This difference was maintained up to 24h post-PCI. Conversely, the mean hsCRP did not significantly differ at the follow-up points for the controls without ISR.
1. INTRODUCTION
Coronary Artery Disease (CAD) is considered the most common cardiovascular disease and is a significant cause of death worldwide [1]. There is a consistent relationship between inflammatory biomarkers and cardiovascular disease [2].
C-reactive Protein (CRP) is a well-established inflammatory biomarker in predicting cardiovascular events. It has been repeatedly shown that high hsCRP levels were positively correlated with major adverse cardiovascular outcomes [3, 4]. Recently, Sonaglioni et al. [5] found that the common carotid artery intima-media thickness was significantly associated with elevated CRP levels in patients with idiopathic pulmonary fibrosis. Moreover, several studies confirmed that CRP elevation was associated with CAD and widespread advanced and unstable atherosclerotic disease, for example, carotid artery disease [6-8]. Yip et al. studied 168 unstable angina patients and found that the hsCRP level at baseline and 180 days after unstable angina correlated with the progression of moderately diseased non-culprit native coronary lesions, not In-Stent Restenosis (ISR) [9].
Statins are well-tolerated medications in most patients [10], and their prescription is increasing steadily, but it is still far below the current guideline recommendations, especially in diabetic patients [11]. Statins were found to reduce the inflammatory markers. Werida et al. in 2012 found that rosuvastatin is more effective than atorvastatin in reducing inflammatory biomarkers, including hsCRP in type 2 diabetics [12]. In JUPITER (Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin trial), a primary prevention trial that studied 17,802 patients with high hsCRP (>2 mg/L) and normal or low Low-Density Lipoprotein Cholesterol (LDL-C). The trial was ended prematurely as rosuvastatin significantly reduced major cardiovascular events after 1.9 years of follow-up compared with the placebo group [13].
Percutaneous Coronary Intervention (PCI) showed morbidity and mortality benefits in a broad spectrum of CAD patients. However, coronary stents cause local and systemic inflammatory reactions mediated by inflammatory cytokines that cause hsCRP release [14, 15]. Coronary ISR is considered an unfavourable outcome post-stent implantation [16] and is thought to occur due to inflammatory reaction and proliferation of smooth muscles within the stent. Even though restenosis mechanisms differ and depend on the type of implanted stent, the primary mechanism is intimal hyperplasia [17].
The present study aimed to investigate the hsCRP inflammatory response to PCI in CAD patients with coronary ISR vs patients without ISR.
2. METHODS
This case-control study was conducted at the cardiology department of the Egypt Air Hospital, Cairo, Egypt. We recruited 80 CAD patients previously treated by PCI and Drug-Eluting Stent (DES) implantation, who later had Coronary Angiography (CAG) due to chest pain or other equivalents symptoms. Based on the results of CAG, the 80 patients were subdivided into 2 groups; group A (n=40) (patients with ISR; >50% stenosis within the stent) and group B (n=40) (controls, patients without ISR). Patients were treated by PCI at the discretion of the treating cardiologist and after discussion with the interventional cardiologist. The hsCRP inflammatory response to the intervention was assessed by measuring hsCRP levels at baseline (pre-PCI) and after 8, 16, and 24 h post-PCI.
We included adult patients of both genders who had Acute Coronary Syndrome (ACS) or stable CAD. The exclusion criteria included patients with acute or chronic inflammatory conditions (e.g., rheumatological diseases) and patients with contraindications to CAG (e.g., severe infection, sepsis, significant haemorrhagic diathesis, or allergy to contrast media).
2.1. Ethical Considerations
This work was approved by the Research Ethics Committee of the Faculty of Medicine, Suez Canal University, Ismailia, Egypt. All enrolled patients signed an informed consent form. Patients were informed that their details would remain confidential.
2.2. Collection of Data
We interviewed the patients to obtain a history that included demographic details like name, age, gender, and address. We also inquired about the risk factors of atherosclerotic CVDs as diabetes mellitus, hypertension and smoking. A complete cardiac examination was also carried out.
2.3. Criteria of Clinical Diagnosis of Risk Factors
A patient was considered hypertensive if she/he was previously diagnosed or on active anti-hypertensive treatment [18]. Patients were diagnosed as having diabetes if the glycated haemoglobin was >6.5%, previously diagnosed or taking antidiabetic treatment [19]. Patients were considered smokers if they smoked >100 cigarettes and were currently smoking. Subjects who smoked ≥100 cigarettes during their lifetime but had not smoked for the last 24 months were classified as ex-smokers. Both ex-smokers and those who never smoked were considered non-smokers [20, 21].
2.4. Diagnosis of Acute Coronary Syndrome (ACS)
We included patients with ACS and those with chronic coronary syndrome. Acute Myocardial Infarction (AMI) requires at least 2 of the following: symptoms suggestive of CAD, ischemic Eectrocardiogram (ECG) findings, and elevated cardiac biomarkers [22]. ECG suggestive of ACS includes presumably new pathologic Q waves or a new left bundle branch block. Moreover, ST-elevation is > 0.1 mV in limb leads or > 0.2 mV in chest leads and is present in 2 or more leads. Unstable angina is diagnosed as presenting with symptoms suggestive of ischaemia without cardiac biomarker elevation, and the ECG might indicate ischaemia [23].
2.5. Clinical and Catheterization Data
All patients underwent a complete clinical examination and an ECG. CAG utilizing the standard technique by femoral access. The decision for PCI was made after a discussion between 2 consultant cardiologists. PCI was carried out as an emergency procedure for those patients with ACS or on an elective basis for those patients with chronic coronary syndrome. Before PCI, all patients received oral loading of aspirin 300 mg and clopidogrel 600 mg once the coronary anatomy was known, and the decision to PCI was taken [24]. During the procedure, 10,000 u of Unfractionated Heparin (UFH) was used; for prolonged PCI (>30 min), another dose of UFH could be used [25]. All PCI procedures were carried out utilizing the same DES (everolimus-eluting-stent).
2.6. Assessment of In-Stent Restenosis (ISR)
We considered ISR if there was a stenosis of >50% in the previous stenting area or within 5 mm from the stent [26].
2.7. Laboratory Data
Blood samples were obtained from all cases and controls to measure hsCRP at baseline (before PCI/CAG), and after 8, 16 and 24 h. Peripheral venous blood was drawn, then serum was frozen at -70°C, and later hsCRP levels were measured using a CRP (latex) high sensitivity immuno-turbidometric method (Roche Diagnostics, USA) using a Roche/COBAS INTEGRA system 400 autoanalyser (Roche Diagnostics, Indianapolis, Indiana, USA), with a measurement range of 0.1 to 20 mg/L. Samples with a concentration below this limit were assigned a value of 0.1 mg/L. Commercial control standards were used for verification of the assay performance.
We defined hsCRP levels <1 mg/L as associated with a low risk of development of Cardiovascular Diseases (CVDs). Values between 1 and 3 mg/L were associated with the average risk and those >3 mg/L values were linked to high risk [27, 28].
2.8. Statistical Analysis
We used the IBM SPSS (Statistical Package for Social Science) program for statistical analysis (version 21.0, Inc., Chicago. IL) for data analysis. Qualitative data are shown as numbers and percentages, and the Chi-Squared test was used. We used Fisher’s exact test when >25% of the table cells have a value <5 for 2x2 qualitative data. Continuous data that were distributed normally presented as mean and standard deviation (SD) and an independent student t-test was done. Pearson’s correlation was used to assess the correlation between hsCRP and the CK-MB. Repeated measures analysis of variance (ANOVA) in the study groups was used to test the difference between the serial hsCRP measurements (baseline, 8, 16 and 24 h post-PCI/CAG). A p<0.05 was considered significant.
3. RESULTS
We recruited 80 CAD patients who had a previously implanted DES (everolimus-eluting stents). Subsequently, they underwent CAG due to chest pain or other related symptoms and were divided into 2 groups. ISR cases group (n=40) included patients with ISR, and the No-ISR control group (n=40) did not have ISR. The baseline characteristics are listed in Table 1. There were no significant differences in age, gender, smoking, hypertension, and diabetes mellitus prevalence between the ISR cases and the controls. The baseline hsCRP was significantly higher in the cases with ISR than the controls: 3.5±1.7 and 2.0±1.5 mg/L, respectively (p<0.0001). Similarly, the hsCRP 24h post-intervention was significantly higher in ISR cases than in the controls (p<0.0001). The cardiac biomarker CK-MB was 151.9±109.3 and 126.5±104.4 IU/L, respectively; the difference was not significant (p=0.29).
The hsCRP levels were cross-tabulated between the 2 study groups in Table 2. The baseline hsCRP level showed a substantial increase in the ISR group compared with the No-ISR. There were 36 (90%) patients of ISR who had a high hsCRP (>3 mg/L) compared with 25 (62.5%) patients in the controls (Fig. 1). Patients with ISR had higher frequencies and percentages of elevated CRP than the No-ISR controls. This difference was maintained at all time points, baseline, after 8, 16, and 24 h (p<0.05).
| Variables |
ISR Group (n=40) |
No-ISR Group (n=40) |
p |
|---|---|---|---|
| Age, years (mean+SD) a | 58.5 ± 5.8 | 56.8 ± 6.9 | 0.24 |
| Women, n (%) b | 15 (37.5%) | 16 (40%) | 0.81 |
| Smokers, n (%) b | 18 (45%) | 15 (37.5%) | 0.49 |
| Hypertension, n (%) b | 19 (47.5%) | 16 (40%) | 0.49 |
| Diabetes mellitus, n (%) b | 20 (50%) | 12 (27.5%) | 0.06 |
| Systolic Blood Pressure (mmHg) a | 132.6±23.8 | 129.5±13.4 | 0.47 a |
| Diastolic Blood Pressure (mmHg) a | 77.4±10.8 | 75.5±11.3 | 0.43 |
| Glucose Level (mg/dL) a | 159±53 | 146±48 | 0.27 |
| Baseline hsCRP (mg/L) a | 3.5±1.7 | 2.0±1.5 | <0.0001* |
| hsCRP (mg/L) at 24 h post-intervention a | 6.7±4.3 | 2.3±1.4 | <0.0001* |
| CK-MB (IU/L) at 24 h post-intervention a | 151.9±109.3 | 126.5±104.4 | 0.29 |
a. Continuous variables have been expressed as mean+SD and assessed by the independent student t-test.
b. Categorical variables are expressed as number (n) and frequency (%) and tested using the χ2 test.
Abbreviations: ISR, in-stent restenosis; No-ISR, no in-stent restenosis; hsCRP, high sensitivity C-reactive protein; CK-MB, creatine kinase-MB isoenzyme.
|
Serial Measurements of hsCRP |
ISR Group (n=40) |
No-ISR Group (n=40) |
p (a) | |
|---|---|---|---|---|
| Baseline (Before PCI/CAG) | hsCRP >3 mg/L | 36 (90%) | 25 (62.5%) | 0.007* |
| hsCRP <3 mg/L | 4 (10%) | 15 (37.5%) | ||
| After 8 h | hsCRP >3 mg/L | 37 (92.5%) | 26 (65%) | 0.005* |
| hsCRP <3 mg/L | 3 (7.5%) | 14 (35%) | ||
| After 16 h | hsCRP >3 mg/L | 37 (92.5%) | 27 (76.5%) | 0.01* |
| hsCRP <3mg/L | 3 (7.5%) | 13 (32.5%) | ||
| After 24 h | hsCRP >3 mg/L | 36 (90%) | 27 (76.5%) | 0.02* |
| hsCRP <3 mg/L | 4 (10%) | 13 (32.5%) | ||
(a) Fisher’s exact test was used to assess the difference between the categorical variables.
Abbreviations: hsCRP, high sensitivity C-reactive protein; ISR, in-stent restenosis; No-ISR, no in-stent restenosis; PCI, percutaneous coronary intervention.
Regarding the levels of hsCRP among the cases with ISR (Fig. 2), a repeated-measures ANOVA (with a Greenhouse-Geisser correction) showed that mean hsCRP differed significantly between time points (p<0.001). Post hoc tests using the Bonferroni correction revealed that CRP increased by an average of 2.6 mg/L after 8 h (p<0.001), then increased by an additional 0.6 mg/L between 8 and 16 h (p=0.7) and then decreased by an additional 0.04 mg/L between 16 and 24 h (p=0.9).
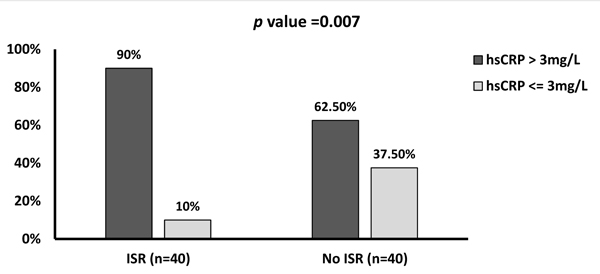
Abbreviations: hsCRP, high sensitivity C-reactive protein; ISR, is-stent restenosis.
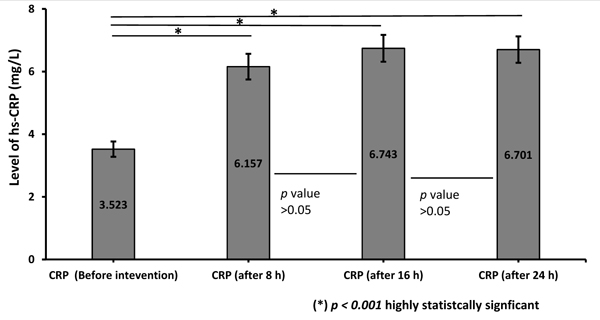
Abbreviations: hsCRP, high sensitivity C-reactive protein; ISR, is-stent restenosis.
Regarding the levels of hsCRP in the controls (Fig. 3), a repeated-measures ANOVA (with a Greenhouse-Geisser correction) showed that mean hsCRP did not differ significantly between time points (p=0.65). Post hoc tests using the Bonferroni correction revealed that CRP increased by an average of 0.2 mg/L after 8 h (p>0.9), then increased by an additional 0.1 mg/L between 8 and 16 h (p>0.9) and then decreased by an additional 0.07 mg/L between 16 and 24 h (p>0.9).
Pearson’s correlation showed that the hsCRP and the CK-MB levels were positively correlated in the ISR group 24h post-PCI (r=0.529, p=0.0004) (Fig. 4).
Most of the study population, 66 (82.5%) patients, had 1-vessel CAD, 13 (16.3%) patients had 2-vessel disease, and only 1 (1.2%) patient had 3-vessel affected. In the 1-vessel CAD, left anterior descending CAD accounted for most of the cases, 46 (57.5%), left circumflex CAD in 7 patients, 29 (72.5%), were managed by DES, followed by balloon-only treatment in 6 (15%) patients, and drug-eluting balloons were used in 5 (12.5%) patients.

Abbreviations: hsCRP, high sensitivity C-reactive protein; ISR, is-stent restenosis.
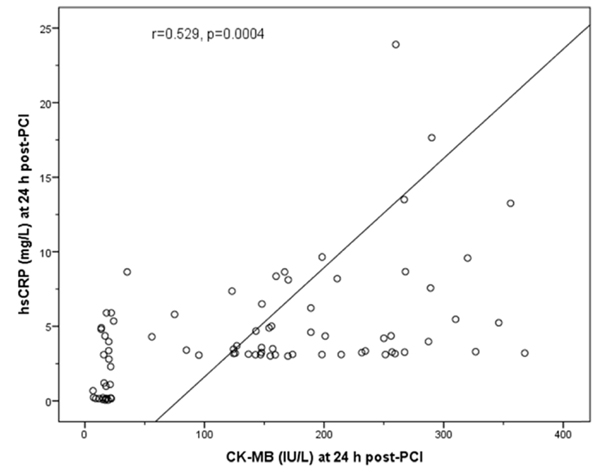
Abbreviations: hsCRP, high sensitivity C-reactive protein; CK-MB, creatine kinase-MB isoenzyme; r, Pearson’s correlation coefficient; PCI, percutaneous coronary intervention.
4. DISCUSSION
CVDs are the major cause of morbidity and mortality worldwide [29]. INTERHEART (A Global Case-Control Study of Risk Factors for Acute Myocardial Infarction) evaluated 9 AMI risk factors which showed significant correlations with AMI [30]. The inflammatory process is highly involved in atherosclerosis and subsequent CAD [31]. Indeed, several studies confirmed the association between the hsCRP and adverse cardiovascular outcomes [5], and there is a significant association between hsCRP and left ventricular dysfunction [32, 33].
The main finding of the present study was that hsCRP levels were substantially higher in the ISR cases than in the controls with patent stents. It was significantly higher at the baseline (pre-intervention) as well as 8, 16, and 24 h post-intervention. For the ISR cases, hsCRP level increased significantly from the baseline levels to 8 h post-procedure, although these levels did not increase significantly from 8 to 24 h. In contrast, the hsCRP in the controls with patent stents did not differ significantly between any measurement points.
A possible explanation for why patients with ISR had higher baseline (pre-intervention) hsCRP levels than the controls is that the ISR cases included more patients with diabetes than the No-ISR controls [20 (50%) vs 12 (27.5%)] although this difference was not significant (p=0.06). In agreement with our findings, Qin et al. [34] conducted a meta-analysis with 21 studies (9,578 patients with 2,667 people with diabetes) and demonstrated a significant association between ISR and diabetes mellitus (OR = 1.70, 95% CI: 1.53-1.89). Furthermore, our cases with ISR showed a higher blood glucose than the controls; however, the difference was not statistically significant. This finding is in agreement with those of Marfella et al. [35], who reported that ISR is positively associated with the blood glucose level.
CRP levels may show marked variability in ACS patients [36]. Interestingly, patients with high CRP levels at baseline showed a hyper-response to different stimuli [37]. This conclusion agrees with our finding that the ISR cases had significantly higher baseline hsCRP and showed a significant increase in the hsCRP during serial measurement post-intervention. In comparison, the no-ISR controls had a lower baseline hsCRP and showed no significant increase in the hsCRP during the serial measurement. Earlier work showed that the CRP variability depends mainly on the baseline CRP and the severity of the different stimuli [37, 38]. The possible aetiology of this response is genetic or acquired [39]. It was stated that myonecrosis could represent the most significant factor and would result in a marked increase of the subsequent CRP [36, 39]. Other proinflammatory stimuli could also be caused by coronary artery intervention [15].
Ren et al. in 2021 [40] conducted a prospective study on 682 patients with ST-elevation myocardial infarction treated by primary PCI. They evaluated the CRP:prealbumin ratio (CPR). After 18 months of follow-up, patients with a high CPR demonstrated higher adverse cardiovascular events than low CPR patients (38.7 vs 12.0%, p<0.01). Similar results were obtained in 1,800 CAD (including stable and unstable angina) patients treated with coronary stents; ISR was positively correlated with an increase in CRP from baseline to peak post-intervention values [41]. Similar findings were reproduced by Saleh and Tornvall, who studied 891 patients with stable and unstable angina with normal troponin T before PCI. CRP was measured before and the day after PCI. A high CRP response to intervention was associated with ISR [42]. Also, our results agree with a meta-analysis of 9 prospective studies that supports a significant relationship between CRP levels at baseline and the subsequent development of ISR [43]. Our work is also consistent with another meta-analysis where 2747 patients received bare-metal stents [44].
Moreover, our results agree with others [45], who showed that serum hsCRP was significantly correlated with the development of ISR. Similarly, our results were consistent with a meta-analysis [46] based on 6 prospective observational studies (1,156 CAD patients) where a high hsCRP predicted the ISR at 6-12 months follow up (odds ratio: 1.16; p < 0.05).
We found that hsCRP was positively correlated with CK-MB, which agrees with another study [47] that included 71 AMI patients.
Niccoli et al. [48] studied the hsCRP level and the Optical Coherence Tomography (OCT) characteristics of neointimal proliferation. They found that patients with high hsCRP levels (>3 mg/L) have a higher restenotic tissue burden that was calculated as restenotic tissue symmetry ratio according to the following equation: (maximum re-stenotic tissue thickness — minimum re-stenotic tissue thickness) / maximum re-stenotic tissue thickness. They did not find a significant relationship between CRP levels and the type of the stent or time of stenting. Furthermore, Hsieh et al. included 1,763 patients and confirmed that high hsCRP (>3 mg/L) after the DES implantation was significantly associated with ISR within 9 months [49].
Segev et al. [50] studied 216 patients with stable CAD with elective coronary artery stents and followed them up for 6 months. There was no significant association between pre-procedural hsCRP or interleukin-6 (IL-6) and restenosis. Their recruitment of only stable CAD without including ACS may explain this difference. It is known that patients with ACS have significantly higher hsCRP than stable CAD patients [51]. Of note, Fong et al. measured the systemic and coronary sinus levels of hsCRP and other inflammatory markers.
In contrast to the above-mentioned evidence, Qin et al. [52] conducted a single-centre prospective study on 1206 Chinese diabetic patients with previous DES implantation and average follow-up of 6 months to 2 years. They found no significant relationship between hsCRP and ISR; they reported an independent relationship between very-low-density lipoprotein (VLDL-C) and ISR. Notably, they studied a small number of patients with ISR group (n=132) vs a relatively large number of controls (n=1074). Moreover, they found a trend toward higher hsCRP in the ISR cases (median hsCRP 2.65 and 2.44 mg/L, respectively), but this difference did not reach statistical significance.
CONCLUSION
hsCRP is significantly associated with coronary in-stent restenosis. ISR patients had a substantially higher baseline hsCRP compared with the controls. Patients with ISR had significantly higher levels of hsCRP 8, 16, and 24 h post-PCI than the No-ISR control group. In contrast, the mean hsCRP did not show a significant difference at all follow-up points for the No-ISR control group.
FUTURE PERSPECTIVES
DES use improved ISR from 25-30% in the era of bare-metal stents to 10-15% in the DES era, but ISR remains a challenge [53]. Many attempts have been made to predict a high risk of ISR. We suggest that large randomized controlled trials are needed to test the hypothesis that the pre-intervention hsCRP could predict the occurrence of ISR. Moreover, sensitivity and specificity should be tested in different populations and ethnicities. Furthermore, there is evidence that several types of miRNA (microRNA) are associated with ISR [54]. We suggest testing miRNA, in addition to hsCRP, in a randomized controlled trial to evaluate their role in predicting ISR and hard cardiovascular endpoints. Moreover, short-term (one week) high dose atorvastatin (80 mg/day) preloading before PCI was associated with lower CRP and IL-6 than the usual dose or reloading dose of atorvastatin in statin-naïve patients with unstable angina [55]. Similarly, a high loading dose of rosuvastatin (40 mg) resulted in lower inflammatory markers, including hsCRP, than the controls without loading dose [56]. Additionally, a high loading dose of atorvastatin (80 mg) added to the standard regimen before primary PCI significantly reduced no-reflow [57] and post-PCI microvascular dysfunction [58], and improved cardiovascular outcomes on 30 days post-PCI [57]. It is unknown whether patients with ISR would benefit from prolonged use of high-dose statin. This is a knowledge gap that will need to be addressed in the future.
STRENGTHS AND LIMITATIONS
ISR is a major clinical problem that needs a lot of work. The aetiology includes several factors, including clinical, patient-related, stenosis-related, and procedural factors. Heightened inflammation has been proposed as a potential cause. It would be helpful to predict the occurrence of ISR. The strength of the current study is that we recruited patients with ISR vs no ISR and found that there was a higher hsCRP in the baseline (before PCI). This would be helpful to predict the occurrence of ISR. Moreover, this study represents real-world results based on Egyptian patients presenting with chest pain after PCI with DES. The findings may provide a relatively feasible way of predicting ISR. However, this is a single-centre study that recruited a small number of patients, and due to the retrospective nature of the study there are certain potential limitations.
LIST OF ABBREVIATIONS
| ACS | = Acute Coronary Syndrome |
| AMI | = Acute Myocardial Infarction |
| ANOVA | = Analysis of Variance |
| BP | = Blood Pressure |
| CAD | = Coronary Artery Disease |
| CAG | = Coronary Angiography |
| CK-MB | = Creatine kinase-MB isoenzyme |
| CPR | = C-reactive protein/prealbumin ratio |
| CRP | = C-reactive protein |
| CVD | = Cardiovascular Disease |
| DES | = Drug-eluting stent |
| DM | = Diabetes mellitus |
| ECG | = Electrocardiogram |
| HbA1c | = Glycated haemoglobin |
| hsCRP | = High-sensitivity C-reactive protein |
| IL-6 | = Interleukin-6 |
| ISR | = In-stent restenosis |
| JUPITER | = Justification for the use of statins in prevention: an intervention trial evaluating rosuvastatin |
| LDL-C | = Low-density lipoprotein cholesterol |
| miRNA | = microRNA |
| No-ISR | = No in-stent restenosis |
| OCT | = Optical coherence tomography |
| PCI | = Percutaneous coronary intervention |
| r | = Pearson’s correlation coefficient |
| SD | = Standard deviation |
| SPSS | = Statistical package for social science |
| UFH | = Unfractionated heparin |
| VLDL-C | = Very-low-density lipoprotein cholesterol |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study has been approved by the Research Ethics Committee of the faculty of Medicine, Suez Canal University, Ismailia, Egypt.
HUMAN AND ANIMAL RIGHTS
All research procedures were followed in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Informed consent was obtained from all participants.
AVAILABILITY OF DATA AND MATERIALS
The data supporting this study is present within the manuscript.
FUNDING
None.
CONFLICTS OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
None.


