All published articles of this journal are available on ScienceDirect.
Prognostic Value of Troponin I in COVID-19 Patients
Abstract
Introduction:
Corona Virus Disease (COVID -19) patients present mainly with respiratory manifestations and viral pneumonia. The cardiovascular system presentation includes early signs of acute myocardial injury. Cardiac troponin I (cTnI) is a gold-standard biomarker for necrosis and myocardial risk assessment.
Aim of the work:
This study aimed to assess the prognostic value of cTnI in COVID-19 patients.
Methodology:
We report a prospective study that included 92 COVID-19 patients admitted to the El Helal Hospital, Sohag, Egypt. Upon admission, routine investigations including cTnI, chest Computed Tomography (CT), and Electrocardiogram (ECG) were carried out. The patients admitted to the intensive care unit (ICU) also had echocardiography.
Results:
More than half of the patients (55.4%) were admitted to ICU; cTnI level was elevated in 30 patients (58.8%), of whom 17 died (56.7%). There were statistically significant differences regarding the relation between cTnI level, D-dimer and the need for ICU admission and death (p=0.001).
Conclusion:
We conclude that cardiac troponin I levels are a prognostic factor for ICU admission and mortality in COVID-19 patients.
1. INTRODUCTION
By the end of 2019, specifically in Wuhan, China, Coronavirus disease (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) firstly emerged [1]. The World Health Organization (WHO) declared COVID-19 as a pandemic on March 11th, 2020 [2].
SARS-CoV-2 is a member of the Coronavirus family, which has spike like proteins attached to ACE 2 receptor cells in different organs like the lung, heart and blood vessels then start to replicate within it [3].
As we know, COVID-19 patients present mainly with respiratory manifestations and viral pneumonia with thecharacteristic ground-glass opacity (GGO) in CT chest; the cardiovascular system presents with early signs of acute myocardial injury [4].
Cardiac Troponin I (cTnI) is a gold-standard biomarker for necrosis in myocardial risk assessment and it is released only in the case of myocardial injury, whatever the mechanism of insult [5].
The primary end-point of the study is the relation of cTnI level and the outcome of patients (whether improved or need to mechanical ventilation or death).
2. MATERIALS AND METHODS
2.1. Study Design
This is a follow up prospective study conducted at El Helal Sohag Hospital, which was established for COVID-19 patients in Sohag Governorate in the period from the 2ndof May to the 10th of July 2020.
All patients included in this study had symptoms of COVID-19 infection and its diagnosis was confirmed by positive PCR nasopharyngeal swab and CT chest with GGO.
2.2. Data Collection
We obtained the clinical data from all patients, including demographic data, history, clinical examination, imaging findings, laboratory results, treatment plan and clinical outcomes. All patients had routine investigations and cardiac troponin I on their admission.
2.3. Laboratory Technique
Seven milliliters (ml) of venous blood was taken from each participant by using sterile gloves under aseptic conditions and then the samples were divided as follows;
1-3ml of blood was put in a plain tube which was left to clot, then the serum was separated. After this, we used a part of it for the assessment of cardiac troponin I(ng/ml).
2.4. Principle
The ST AIA-PACK c TnI 3rd -Gen is a two-site immunoenzymometric assay which is performed on TOSOH AIA System analyzer in the ST AIA PACK cTnI 3rd Gen test cups. Cardiac troponin I in the test sample is bound to the monoclonal antibody immobilized on magnetic beads and enzyme-labeled monoclonal antibody in the test cup. The magnetic beads were washed to remove the unbound enzyme labeled antibody and then incubated with a fluorogenic substrate, 4-methylumbelliferyl phosphate (4-MUP). The amount of the enzyme-labeled monoclonal antibody bound to the beads was directly proportional to the cTnI concentration in the test sample. A standard curve was constructed and the unknown sample concentrations were calculated using the curve.
1.8-2ml of the sample was collected in 0.2 ml sodium citrate for the assessment of D-Dimer (ug/ml).
The ST AIA-PACK D-Dimer is a two-site immunoenzymometric assay which is performed on a TOSOH AIA System analyzer with the same principle as cardiac troponin I.
2-3ml sample was used on EDTA blood for assessment of complete blood count (CBC) by CELL-DYN 3700 (Abbott Laboratories, Diagnostic Division, IL, USA] [6].
2.5. Statistical Analysis
Data collected regarding history, basic clinical examination, laboratory investigations and outcome measures were coded, entered and analyzed using Microsoft Excel software. The data collected were tabulated and analyzed by SPSS (statistical package for social science) version 20 (Armonk, NY: IBM Corp) on IBM compatible computer. The data were tested for normality using Kolmogorov–Smirnov test. Descriptive statistics of qualitative data are represented as number and percentage, quantitative group is represented by mean ± SD. Analytic statistics included independent samples’ Student t-test; it is a test of significance used for comparison between two groups having quantitative variables with a normal distribution. A P-value of < 0.05 was considered statistically significant.
3. RESULTS
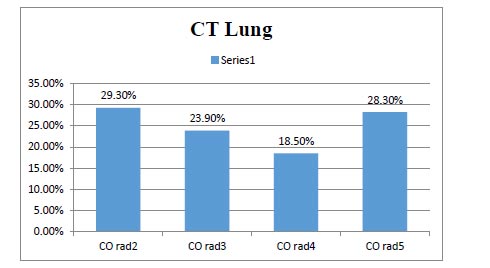
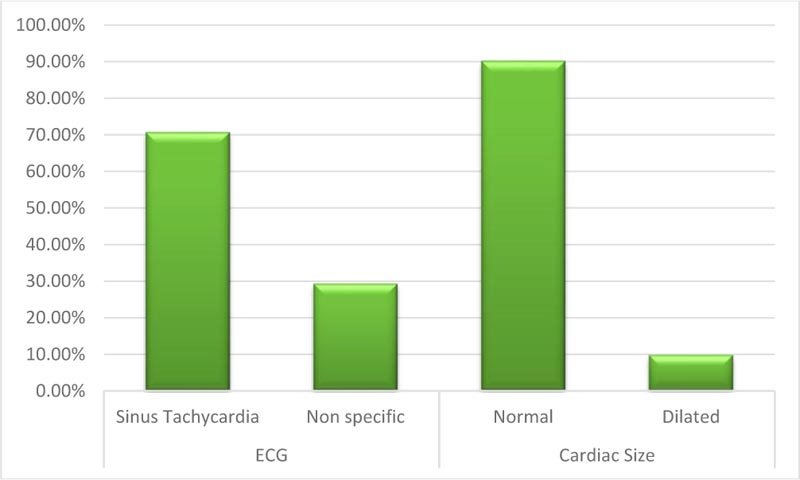
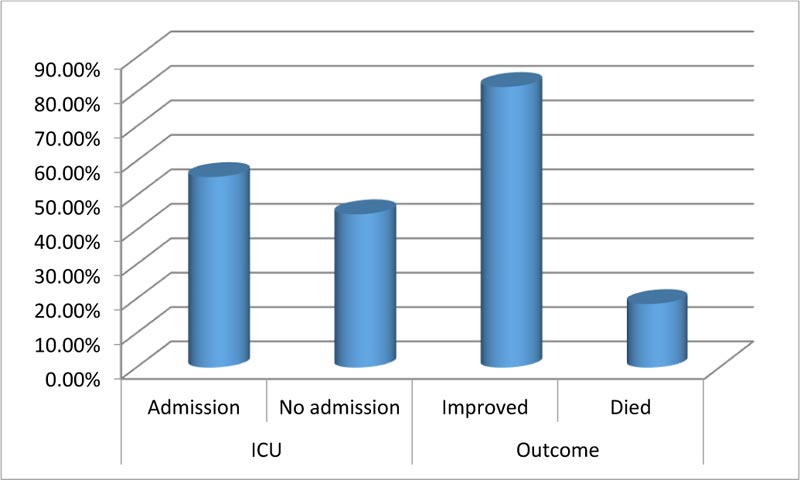
This study included 92 patients. Upon admission, routine investigations including troponin I, CT chest, ECG and (Echocardiography for patients admitted at ICU) were performed . As shown in Table 1, the mean age of the included patients was (54.9) with SD (11.27), about (49%) of them were males, most of them presented with general and respiratory manifestations as fatigue, tachycardia, cough and tachypnea (92%, 84%, 83% and 77%) and more than half of them (52%) were suffering from another comorbidity as (Diabetes Mellitus). With regard to CT chest findings as shown in Fig. (1), about one-third of the patients (28%) were having Co rad 5. Fig. (2) displays that 70% of the patient had sinus tachycardia in ECG findings. Fig. (3) indicates that more than half of the patients (55.4%) were admitted to ICU, and about (18%) of the whole studied patients (17/92) had died. Table 2 shows that there were statistically significant differences with regard to the relation between troponin I level, D- dimer and the need for ICU admission and death with p-value (P<0.001)
4. DISCUSSION
Results of this study revealed that more than half of the patients, 51 of 92 (55.4%) were admitted to ICU, and Troponin I was elevated in 30 patients of 51 (58.8%), and seventeen patients had died of 30 patients with elevated cTnI (56.6%). This was in agreement with Qing et al., who found the presence of myocardial injury in COVID-19 patients during hospitalization and (12.5%) of the patients had presented abnormalities similar to myocarditis, with an increase in cardiac troponin I especially during hospitalization also in about (37.5%) of them, especially in those who were died. Cardiac troponin levels were significantly increased one week before death. With normal findings in Echo and ECG, they suggested that the increase in troponin was related to systemic disorders and could be the warning sign for the death of patients with COVID-19 and should be taken seriously in clinical practice [7].
| Characteristics | Statistical Analysis |
| Age | Mean (54.9),SD(11.27) |
| Gender | |
| Male | N (%) 49 (53.3%) |
| Female | N (%) 43(46.7%) |
| Symptoms& Signs | |
| Temperature | Mean ± (SD) 38.337 ± (0.55) |
| Cough | N (%) 83 (90.2%) |
| Dyspnea | N (%) 59 (64.1%) |
| Chest Pain | N (%) 0 (0%) |
| GIT | N (%) 33 (35.9%) |
| Fatigue | N (%) 92 (100%) |
| Loss of smell, Taste | N (%) 27 (29.3%) |
| RR Normal | N (%) 15 (16.3%) |
| Tachypnea | N (%) 77 (83.7%) |
| HR Normal | N (%) 8 (8.7%) |
| Tachycardia | N (%) 84 ((91.3%) |
| Comorbidity | |
| COPD | N (%) 25 (27.2%) |
| DM | N (%) 52 (56.5%) |
| HTN | N (%) 50 (54.3%) |
| IHD | N (%) 17 (18.5%) |
| AF | N (%) 19 (20.7%) |
| Total | 92 |
| - | ICU | Mean± SD | P value | |
|---|---|---|---|---|
| D dimer | Admission | 51 | 2117.07±1181.03 | P<0.001 |
| No admission | 41 | 126.09±168.63 | ||
| Troponin I | Admission | 51 | 5.39±4.70 | P<0.001 |
| No admission | 41 | .017±0.015 | ||
| - | Outcome | - | - | |
| D dimer | Improve | 75 | 388.67±558.98 | P<0.001 |
| Died | 17 | 2783.3±1035.32 | ||
| Troponin I | Improved | 75 | 0.27±.76 | P<0.001 |
| Died | 17 | 8.46±3.66 | ||
This was also similar to the findings shared by Zhou et al., who found that there was an increase in mortality rate by (50%) of COVID-19 patients with myocardial injury (raised serum cardiac troponin I levels] [8].
Wang et al., published that about (25%) of the patients in the intensive care unit (ICU) had a previous cardiovascular disease (CVD) and (58%) of those patients were hypertensive [4].
This also was similar to our finding that (56.5%) of the studied patients had DM, (54.3%) of them had hypertension, about (18.5%) of patients had IHD and (20.7%) of them had atrial fibrillation.
There were statistically significant differences regarding the relation between troponin I level, D- dimer and the need for ICU admission and death with p-value (p <0.001).
, Huang et al., reported elevation of cTnI (>28 ng/mL) in 5 out of 41 (12%) of COVID-19 patients. All 5 then developed an acute myocardial injury, and 4 out of these 5 were admitted to the ICU [1].
Recent data has shown that cTnI levels are mildly elevated in all patients with SARS-CoV-2 infection, but cTnI levels are markedly elevated in patients with severe SARS-CoV-2 infection compared to those with milder forms of the disease [9].
CONCLUSION
From this study it was concluded that cardiac troponin I has predictive potential for severe morbidity in COVID-19 patients.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study has been approved by Research Ethics Committee Medicine of Sohag University.
HUMAN AND ANIMAL RIGHTS
Not applicable.
CONSENT FOR PUBLICATION
Informed consent was obtained from all participants.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
None.
CONFLICTS OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


