All published articles of this journal are available on ScienceDirect.
Myocarditis in a Young Patient with Celiac Disease; A Case Report and Literature Review
Abstract
Myocarditis has numerous aetiologies, and Celiac Disease (CD) has been described as a rare cause. CD has received little attention in current guidelines and may be underdiagnosed. We report a case involving a 28-year-old male with myocarditis causing severe left ventricular dysfunction and dilatation that was probably related to CD. This case highlights the importance of screening for CD in patients presenting with myocarditis and signs of malabsorption when other common causes are excluded. Besides optimal medical treatment and cardiac resynchronization therapy, a gluten-free diet and immunosuppression may also be effective measures in the management of CD-related myocarditis.
1. INTRODUCTION
Myocarditis is an inflammatory disease of the myocardium caused by a variety of infectious and non-infectious conditions such as direct toxicity or immune-mediated drug reactions and systemic autoimmune disorders [1, 2]. Generally, the disease is self-limiting. However, in some patients, severe/fulminant myocarditis may lead to dilated cardiomyopathy and severe Left Ventricular (LV) dysfunction, a feared complication [2]. Although current guidelines [1] mention autoimmune disease as a cause of myocarditis, Celiac Disease (CD) has received little attention in the literature. However, CD was found in 4.4% of 187 patients with myocarditis [3] and 5.7% of 52 patients with dilated cardiomyopathy [4]. This suggests that CD may be underdiagnosed. In the present article, we highlight the importance of screening for CD in a young patient with severe systolic LV dysfunction.
2. CASE REPORT
A 28-year-old male refugee from the Middle East was referred to our hospital with a 3-month history of increasing dyspnoea, initially on exertion (New York Heart Association [NYHA] functional class III), but at the time of presentation at rest. He had intermittent chest tightness, which had no relation with exercise, respiration, cough, or food intake. He also experienced intermittent palpitations lasting for up to 3 h. His family history was unremarkable with regard to premature coronary artery disease, cardiomyopathy, or sudden cardiac death. He did not smoke, had no history of alcohol or other substance abuse, and did not take any medications. He had not experienced any weight loss or night sweats but occasionally experienced cough with blood-stained sputum.
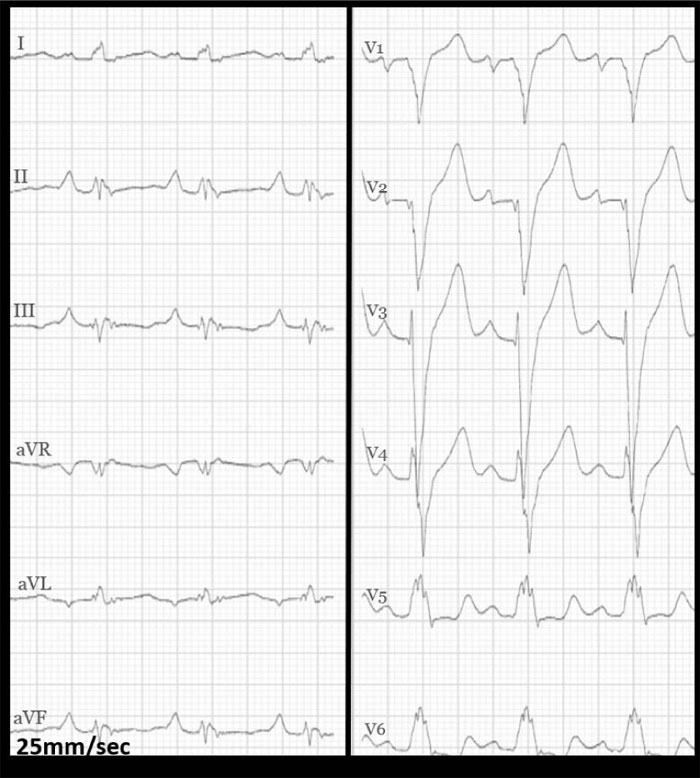
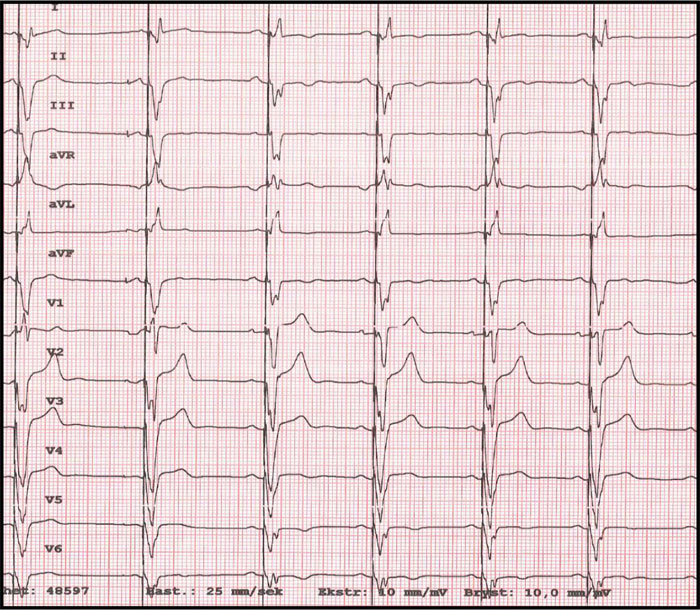
On examination, the blood pressure was 127/66 mmHg, heart rate was 95/min, oxygen saturation was 100% at room air, and the temperature was 37.1°C. Auscultation of the lungs and heart was normal. The jugular venous pressure was normal, and there was no peripheral oedema. The chest x-ray showed a slightly enlarged cardiac shadow and a sign of interstitial oedema. The CT scan of the thorax showed mediastinal lymphadenopathy, ground-glass opacities in the right lower lobe, and a non-specific nodule in the right upper lobe. Induced sputum for acid-fast rods and QuantiFERON-TB (interferon-Gamma Release Assay used in testing for tuberculosis infection) were both negative, excluding pulmonary tuberculosis. Blood tests showed mild iron deficiency anaemia with a haemoglobin 10.9 g/dL (normal range: >13.4 g/dL), mean corpuscular volume 73fL (normal range: 82-98 fL), ferritin 32 μg/L (normal range: 34-300 μg/L), and increased levels of serum transferrin receptor at 12.1 mg/L (normal range: 2.2-5 mg/L). Transferrin receptor is a carrier protein for transferrin, needed for the import of iron into the cell, and is upregulated in response to low intracellular iron concentration. There was elevation of the C-reactive protein level at 31 mg/L (normal range: <5 mg/L), troponin T level at 57 ng/L (normal range: <15 ng/L) and pro-brain natriuretic peptide (pro-BNP) at 690 ng/L (normal range: <85 ng/L). The serum creatinine level was normal at 81 μmol/L (normal range: 60-105 μmol/L). The ECG (Fig. 1) showed sinus rhythm with left bundle branch block.
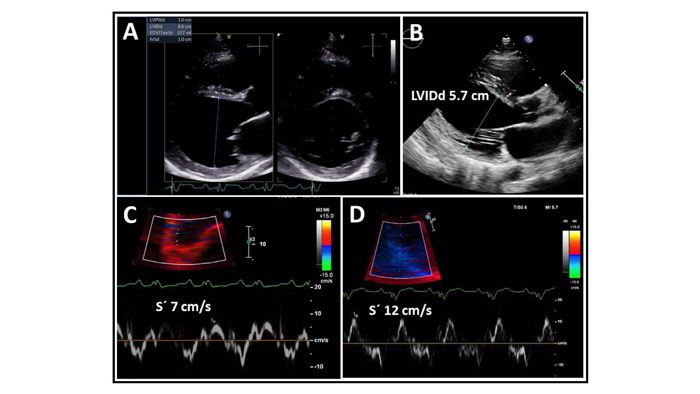
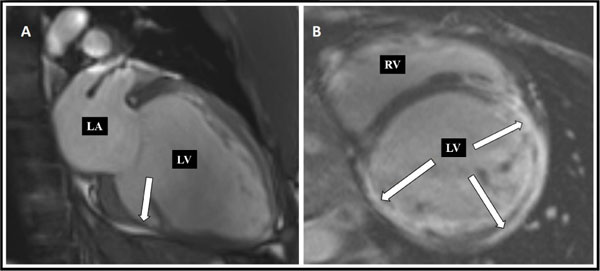
Standard transthoracic echocardiography on admission revealed a dilated LV with an end-diastolic dimension of 6.6 cm (Fig. 2), global hypokinesis with LV ejection fraction (LVEF) of 25%, and mild mitral regurgitation. Coronary CT angiography showed normal coronary arteries with a calcium score of zero. Cardiac magnetic resonance (MR) demonstrated extensive subepicardial contrast uptake and oedema as a sign of ongoing inflammation, as well as dilation of the LV with myocardial scarring and thinning, as a sign of chronicity, and highly suggestive of myocarditis (Fig. 3).
The patient was started on heart failure medications, and an extensive workup was initiated to determine the aetiology of the myocarditis.
Common viral and bacterial serology such as Parvovirus B-19, Cytomegalovirus, Epstein-Barr virus, Adenovirus, Herpes simplex virus, Varicella zoster virus, hepatitis B virus, hepatitis C virus, human immunodeficiency virus, Lyme disease, and syphilis were negative. Erythrocyte sedimentation rate (ESR) was normal: 9 mm/h (normal range: 1-15 mm/h), and antinuclear antibodies (ANA), antineutrophil cytoplasmic antibodies (ANCA), and rheumatoid factor were also negative. A fluoroscopy-guided endomyocardial biopsy of the right ventricular septum was performed through the right internal jugular vein. The biopsy showed focal fibrosis and sparse lymphocytic inflammatory infiltration.
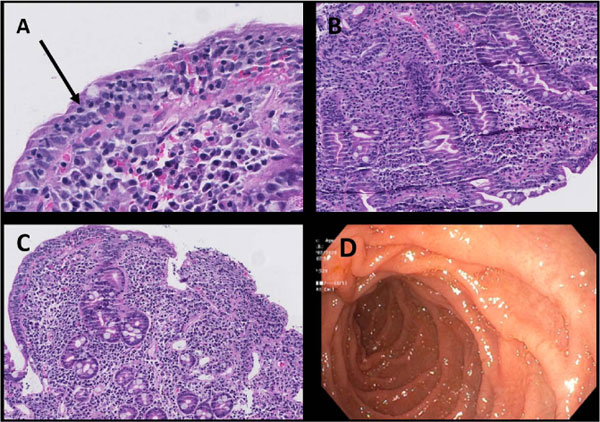
Iron deficiency anaemia, uncommon in young males, aroused the suspicion of a possible malabsorption syndrome. Upon further questioning, he reported some abdominal discomfort and bloating after intake of certain foods. He was subsequently tested for anti-tissue transglutaminase IgA which was strongly positive at >250 U/ml (normal range: <14.9 U/ml), and Anti-deamidated gliadin IgG which was also positive: 16 U/ml (normal range: <14.9 U/ml). Upper gastrointestinal endoscopy revealed a normal macroscopic appearance, except for slight oedema in the proximal duodenum (Fig. 4). Small intestinal biopsies were taken from the distal part of the duodenum during endoscopy and placed in 4% buffered formalin for routine histological analysis. Duodenal histology showed severe villous atrophy with crypt hyperplasia and an increased number of intraepithelial lymphocytes as well as inflammation in the lamina propria (Marsh type 3c lesion) [5], consistent with CD (Fig. 4a-c).
The patient was started on a gluten-free diet and referred to the CD outpatient clinic for follow up. After a thorough workup, his dilated cardiomyopathy was believed to be caused by myocarditis, secondary to the CD. Three weeks after discharge, heart failure treatment with sacubitril/valsartan, metoprolol, and furosemide was optimized. The patient initially did not wish to receive Cardiac Resynchronization Therapy (CRT). At 4-month follow-up, he reported clinical improvement and claimed good compliance with a gluten-free diet. His breathlessness now occurred only on severe exertion, and he no longer had chest pain. The haemoglobin level had normalized, but he was still mildly iron deficient. The pro-BNP and inflammatory parameters had improved. The LVEF had increased to 35% compared with 25% at his initial admission, and the LV end-diastolic dimension was 5.7 cm compared to 6.6 cm on admission. The septal mitral annular S’ was 12 cm/s compared with 7 cm/s on admission (Fig. 2). Cardiac MR after 6 months showed extensive scarring but no sign of active inflammation. At 6 months follow-up, the patient received a CRT-D device resulting in narrowing of the QRS complex to 150 ms (Fig. 5).
3. DISCUSSION
Myocarditis is an inflammatory disease of the myocardium caused by a variety of infectious (mostly cardiotropic viruses) and non-infectious conditions such as direct toxic or immune-mediated reaction to drugs and systemic autoimmune disorders. Generally, it is self-limited, but some patients may experience a severe/fulminant course of the disease with dilated cardiomyopathy, severely reduced LVEF, and arrhythmias.
Our patient presented with myocarditis that was perceived as an extra-intestinal manifestation of CD. However, it is important to highlight that given the high prevalence of both disorders, there is also a possibility that these two conditions may coexist independently of each other. Some [3, 4, 6], but not all [7] studies have shown a relationship between CD and myocarditis, with a prevalence ranging from 1.8-5.7% [3, 4, 6]. There have also been several case reports of CD-related myocarditis [8-15]. CD is an autoimmune disease [16], and extra-intestinal manifestations have been described in almost all organs [17]. In patients with untreated CD, IgA serum antibodies directed against heart muscle structures are present [18], suggesting an autoimmune process leading to myocardial injury. These antibodies are not seen in CD treated or control groups [18].
Similar to our case, Frustaci et al. [3] showed that 9 of 13 patients with CD and myocarditis had iron deficiency anaemia as the only clinical suggestion of CD.
CONCLUSION
We, therefore, recommend that patients with myocarditis and iron deficiency anaemia or other signs of malabsorption should be screened for CD, even in the absence of obvious intestinal symptoms. Besides optimal medical treatment of heart failure and CRT, a gluten-free diet and immunosuppression can also be effective [3, 9].
LIST OF ABBREVIATIONS
| CD | = Celiac Disease |
| CRT-D | = Cardiac Resynchronization Therapy Defibrillator |
| EF | = Ejection Fraction |
| LV | = Left Ventricle/Ventricular |
| LVEF | = Left Ventricular Ejection Fraction |
| Pro-BNP | = Pro-Brain Natriuretic Peptide |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The author confirms that the requirement for ethical approval is waived off by the Department of Heart Disease, Haukeland University Hospital, Norway.
HUMAN AND ANIMAL RIGHTS
Not applicable.
CONSENT FOR PUBLICATION
Informed consent was obtained from the patient.
STANDARD OF REPORTING
CARE guidelines and methodology were followed.
FUNDING
None.
CONFLICT OF INTEREST
The authors have no disclosures related to this manuscript.
ACKNOWLEDGEMENTS
The authors thank Dr. Hege Sætran for histological images from duodenal biopsies with interpretation and Professor John B. Chambers for valuable comments and suggestions during the preparation of the manuscript.


