All published articles of this journal are available on ScienceDirect.
Cardiac Dual-source Computed Tomography for the Detection of Left Main Compression Syndrome in Patients with Pulmonary Hyper-tension
Abstract
Introduction:
Left Main Compression Syndrome (LMCS) represents an entity described as the extrinsic compression of the left main coronary artery (LMCA) by a dilated pulmonary artery (PA) trunk. We examined the presence of LMCS in patients with pulmonary hypertension (PH) using dual-source computed tomography (DSCT), as a non-invasive diagnostic tool.
Methods:
The following parameters were measured: PA trunk diameter (PAD), the distance between PAD and LMCA (LMPA) and the distance between PA and aorta (AoPA). These measurements were related with demographic, echocardiographic, hemodynamic and clinical parameters. Angiography was performed in two patients with LMCS suspected by cardiac computed tomographic angiography. Patients without PH but with angina were examined as controls, using DSCT cardiac angiography to assess the same measurements and to detect the prevalence of coronary artery disease.
Results:
PA diameter value over 40.00 mm has been associated with PH and LMCS. Furthermore, LMCS did not occur at a distance smaller than 0.50 mm between the PA and the LMCA, and did not correlate with the distance between the PA and the aorta or with cardiac index and NT-proBNP.
Conclusion:
DSCT may represent the initial testing modality in PH patients with dilated PA trunk to exclude LMCS. A periodical rule-out of this rare entity, as assessed by DSCT, in patients with a severely dilated PA seems to be mandatory for PH patients contributing to survival improvement.
INTRODUCTION
Left main compression syndrome (LMCS) is characterized by extrinsic compression of the left main coronary artery (LMCA) due to enlarged pulmonary artery (PA) trunk. It is predominantly present in congenital heart diseases or in idiopathic pulmonary arterial hypertension (PAH) [1-4]. In the presence of significant dilatation of the main PA from any etiology, functional and/or anatomic studies should be performed to exclude LMCS [4], sincethe likelihood of LMCA compression in patients with pulmonary hypertension (PH) is positively related to PA [3]. Cardiac computed tomographic angiography is a useful non-invasive tool for screening, even though classical coronary angiography (CCA) is the gold standard for the final diagnosis [5]. In case of the syndrome detection, the optimal therapeutic approach is debatable [6], and is based more on the LMCA stenosis severity than on the objective demonstration of myocardial ischemia [7]. The aim of our study was to examine the presence of LMCS in patients with PH, using dual-source computed tomography (DSCT), as a non-invasive diagnostic tool.
MATERIALS AND METHODS
Study Design
Subjects were enrolled at our institution from September 2011 to June 2012. Subjects were divided into two study groups. The first group included patients 18 to 99 years of age, suffering from PH with regular follow up and recent hemodynamic evaluation by right heart catheterization (RHC). In the first group we recruited 16 PH patients, 3 men and 13 women, who prospectively underwent DSCT pulmonary and coronary angiography. Diagnoses included idiopathic PAH (n:4), connective tissue disorder (n:3), scleroderma (n:2), and chronic thromboembolic disease (n:7). The most recent findings on RHC, transthoracic echocardiography (TTE) and clinical parameters, were retrieved from patients’ files. DSCT pulmonary and coronary angiography was performed for the evaluation of PA dilatation and LMCS diagnosis. The second group (control group) consisted of 16 patients who underwent DSCT angiography with contrast medium during the study period, in order to evaluate chest pain. The study group was enrolled, and matched on body surface area (BSA). All patients underwent a TTE for PH exclusion. PH was excluded if Doppler-calculated pulmonary artery systolic pressure (PASP) at rest was lower than 50 mmHg, based on tricuspid regurgitation. None of the control patients was known to have cardiopulmonary disease and some of them received treatment for arterial hypertension or dyslipidemia. Retrospective reading of examinations is under the approval of the ethics committee in our institution, and does not require further institutional review board approval.
Imaging Assessment and Interpretation
DSCT coronary angiography (Siemens Definition) was performed in all patients and controls. The scanning parameters were as follows: tube voltage 120 kV, effective mAs 500-600, detector collimation 0.60 mm, slice thickness 0.75, rotation time 0.33 s and reconstruction interval at 0.50 mm. Image acquisition was synchronized to the cardiac rhythm by retrospective ECG-gating and for radiation dose reduction ECG pulsing technique was used. The measurements were carried out three times by two blind-folded, experienced radiologists, in order to assess the intra and inter-observer variability. We estimated the mean value for each observer (1st or 2nd), but also a total mean value for each parameter.
Then, two radiologists, blinded to the clinical and hemodynamic data, independently reviewed the DSCT measurements. In order to assess the distance between the PA and the LMCA, measurements were carried out in multi-planar reconstructed images. In addition, pictures reconstructed by volume rendering technique were used for an initial visual assessment of the proximity between the PA and the LMCA (Fig. 1A). The following parameters were estimated: 1) transverse diameter of the PA, 3 cm above the root, (PAD), (Fig. 1B), 2) distance between the PA and the LMCA in coronal view (LMPA), (Fig. 2A), and 3) distance between the PA and the aorta in coronal view (AoPA) (Fig. 2B).
Clinical and Hemodynamic Assessment
For the first group of subjects we assessed the following clinical and hemodynamic characteristics: 1) World Health Organization (WHO) classification, 2) six minute walk test (6MWT) distance, 3) mean PA pressure assessed by RHC, 4) PASP by TTE, 5) cardiac index (CI) provided by RHC, 6) duration of the disease (months), and 7) N-terminal pro-Brain Natriuretic Peptide (NT-proBNP). None of the PH patients complained of angina. All control patients reported thoracic pain, without previous history of coronary artery disease (CAD). Among risk factors for CAD, arterial hypertension and dyslipidemia were taken into account in the inclusion criteria. History of CAD was an exclusion criterion, as were the detection of PH TTE or treatment with diuretics. Each subject’s BSA was calculated. The second study group was enrolled, and matched on BSA. BSA was calculated using the formula BSA =(W 0.425 x H 0.725) x 0.007184, were W stands for body weight in kilograms, and H for body height in centimetres. PH in the first patient group was defined as a value of mean PA pressure equal or greater than 25 mmHg, in RHC. For the second group, PH was defined as a tricuspid regurgitation velocity greater than 3.4 m/sec on the same day DSCT was performed. For patients with LMCS in DSCT angiography, CCA was performed. A 45o left anterior oblique view with 30o cranial angulation was the view LMCS was visualized.
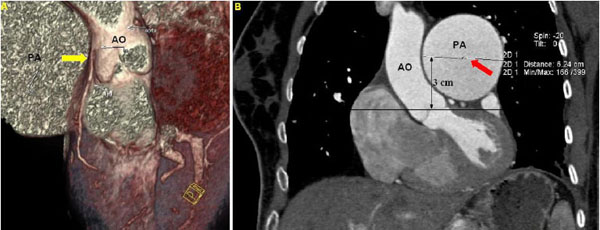
A. Cardiac computed topographic angiography with volume rendering technique demonstrating the proximity between the pulmonary artery and the left main coronary artery (yellow arrow). B. Measurement of transverse diameter of pulmonary artery, 3 cm above the aortic root (PAD – red arrow). (AO: Aorta, PA: Pulmonary Artery, LMCA: Left Main Coronary Artery).
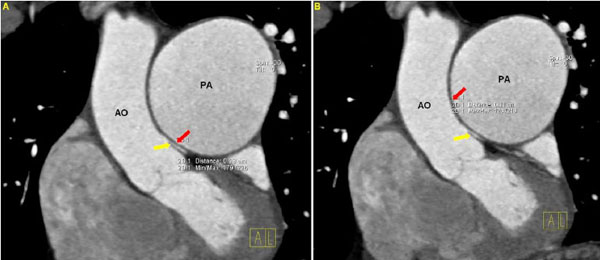
A. Distance between the pulmonary artery and the left main coronary artery (yellow arrow) in coronal view (LMPA – red arrow). B. Distance between the pulmonary artery and aorta in coronal view (AoPA – red arrow). (AO: Aorta, PA: Pulmonary Artery, LMCA: Left Main Coronary Artery).
Statistical Analysis
For the first study group, population clinical characteristics, 6MWT distance, hemodynamic parameters assessed by RHC, PAH specific drug therapy, and NT-pro BNP were analyzed. For both study groups, DSCT measurements were also analyzed. Non-parametric statistics were used due to the small group size. For the same reason, median and inter-quartile range (IQR, 75th-25th percentiles) were used instead of mean and standard deviation. For the comparison between the two categories of a continuous variable, Mann-Whitney U statistics was used. In order to test for potential associations between categorical variables, Fisher’s exact test was applied. Bivariate correlations between continuous variables were tested using Spearman’s rho correlation coefficient. It should be noted that in some categorical variables, the size of one category was too small to extract safe conclusions. P-value was considered significant if p<0.001, due to the inflation of Type I error as a result of many multiple comparisons. Data were analyzed using STATATM (Version 9.0, Stata Corporation, Colleage Station, TX 77845, USA).
RESULTS
The study groups consisted of 32 patients, 13 men and 10 women from the first and second group respectively, with age of 55.00 years (±14.00) for the first group and 57.50 years (±14.50) for the second one. The subject demographics, including PH classification for the first study group, are presented in Table 1. The DSCT measurements provided consist of the median value of the measurements from the two different radiologists.
Patients’ characteristics.
| Group I: PH | Group II: Controls | |
|---|---|---|
| Number/women | 16/ 13 | 16/ 10 |
| Age (median, y) | 57.50 (±14.50) | 55.00 (± 14.00) |
| PH classification | IPAH:4, CTEPH:7, CTD:3, Scleroderma:2 | |
| WHO classification | IV:2, III:7, II:2 | |
| PAH drug therapy | monotherapy:5, combination:8 | |
| PAP mean (mmHg) | 62.00 ± 38.00 | |
| PASP/echo (mmHg) | 87.00 ± 50.00 | 20.00 ± 10.00 |
| PASP/RHC (mmHg) | 80.00 ± 40.00 | |
| CI (l/min/m2) | 2.10 ± 0.60 | |
| NT-proBNP (pg/ml) | 692.50 ± 2125.50 | |
| 6MWD (m) | 377.50 ± 183.00 |
Clinical and Hemodynamic Characteristics for PH Patients
The median time period of PH diagnosis for the first group was estimated at 4.50 years (±10.00) and the median follow up for PH patients was 125.50 weeks (± 124.00). RHC and DSCT were performed in a mean of 30 days apart (median 15 days). The median value of mean PA pressure was estimated at 62.00 mmHg (±38.00) and of the CI at 2.10 l/min/m2 (±0.60). The median PASP measured by TTE was 87.00 mmHg (± 50.00). The median WHO classification was class 2.00 (±1.50), the median 6MWT distance 377.50 m (± 183.00), and the median NT-pro BNP was estimated with a mean value of 692.50 (±2125.50) pg/ml (UL: 184 pg/ml). In two patients with LMCS, CCA revealed stenosis over 70% in LMCA, eccentrically narrowed and inferiorly displaced relative to the coronary cusp. Underlying atherosclerosis was excluded using intravascular ultrasound method (IVUS).
DSCT Measurements
A comparison of the measurements provided by DSCT, as signs of PH revealed significantly higher measurements of PA, lower AoPA and significantly lower measurements of LMPA in the PH group. Inter-observer variability in measurements within each team was less than 5%. Measurements for PH patients had the following measurements: Median PAD was estimated at 33.93 mm (±11.08), the median LMPA 0.67 mm (±0.52) and the median AoPA 1.14 mm (± 0.84). The comparison with the second group is shown in Table 2. The median LMPA was measured at 0.67 mm (± 0.52) for PH group patients and at 1.91 mm (± 3.05) for the control group.
LMPS and Correlations
LMCA compression was observed in two patients of group 1. The first one suffered from idiopathic PAH and the second patient from chronic thromboembolic PH, and both of them had LMPA < 0.50 mm. PA was measured at 45.00 and 62.40mm, AoPA at 1.10 mm and 0.50 mm and LMPA was estimated at 0.40 mm and 0.30 mm for the first and the second patient respectively. The mean PA pressure by RHC was 70.00 mmHg for the first one and 62.00 mmHg for the second, the CI 2.10 l/min/m2 for both of them, the PASP by TTE was 120.00 mmHg and 87.00 mmHg, the 6MWT distance 360.00 m and 432.00 m, and the functional class was WHO II for both patients. The median period of PH diagnosis time for the two patients with LMCS was 7.50 years (±9.00) and the patients were on double combination therapy with PAH-specific drugs. Both of them underwent CCA and LMCS was verified.
Dual source computed tomography measurementS for both of groups I and II (median values).
| Parameter | Group I: PH | Group II: Non-PH | p-value |
|---|---|---|---|
| PA diameter (mm) | 33.98 | 23.38 | <.001 |
| LMPA (mm) | 0.67 | 1.91 | .020 |
| AoPA (mm) | 1.14 | 1.87 | .019 |
Correlations Between Patients Characteristics and LMPA Smaller than 0.50 mm
Six patients were identified with LMPA smaller than 0.50 mm (group III). Significant differences were demonstrated between group III and patients with LMPA equal or greater than 0.50 mm (group IV) (Table 3). The most significant differences between these two groups of patients are the following: The median WHO functional class was 3.00 and 2.00 (p:0.023), the mean duration of PH diagnosis was 6.5 years and 2.00 years (p:0.101), the 6MWT distance was 315.00 m and 456.00 m (p:0.051), the PASP by TTE was 91.00 mmHg and 60.00 mmHg (p:0.953), and the PAD was estimated at 41.27 mm and 32.63 mm (p:0.083) for patients with LMPA smaller than 0.50 mm and those with LMPA greater than 0.50 mm, respectively. Significant correlation was demonstrated between PAD and PASP by TTE (p: 0.079), NT-proBNP value (p: 0.031), and CI by RHC (p: 0.010).
Characteristics comparing group III patients and patients with LMPA>=0.5mm.
| Parameter | Group III | Patients with LMPA>=0.5mm | p-value |
|---|---|---|---|
| Years since diagnosis | 6.50 | 2.00 | .101 |
| WHO classification | II-III | I-II | .023 |
| 6MWD (m) | 370 | 480.50 | .129 |
| Mean PAP (mmHg) | 66 | 61 | .953 |
| CI (l/min/m²) | 2.10 | 2.18 | .906 |
| NT-proBNP (pg/ml) | 817.00 | 692.50 | .828 |
| PA (mm) | 41.27 | 32.63 | .083 |
| AoPA (mm) | 1.09 | 1.26 | .515 |
DISCUSSION
Extrinsic compression of LMCA represents a complication in PH patients associated with sudden cardiac death [8, 9], myocardial infarction and angina [10]. Chest pain in PAH patients is a frequent complain, predominantly attributed to right ventricular (RV) ischemia due to RV dilatation and hypertrophy [11, 12]. Angina is also associated with the LMCA stenosis in case of compression by the enlarged PA, thus appropriate diagnostic evaluation is needed for LMCS exclusion. Delayed presentation of such patients is unusual but some cases of acute coronary syndrome [13] or cardiogenic shock [14], on the basis of LMCS have been described. However, angina is not always present or it may be misdiagnosed as the result of RV ischemia, so LMCS must be excluded in case of angina but also in case of a severely dilated PA.
PA over 40.00 mm has been associated with PH and LMCS [3], and our study confirmed these results that already exist in the literature. Specifically, this PA diameter value was observed to be present in our study as the median PAD in LMCS patients was 51.74 mm. Furthermore, a novel finding in our study was that LMCS did not occur at a distance smaller than 0.50 mm between the PA and the LMCA, with evident implications for LMCS detection strategy. Another novel finding of our study was that LMCS is not correlated with the distance between the PA and the aorta or with CI and NT-proBNP. On the contrary, tendency of a correlation exists between LMCS and duration of disease and PASP.
The limited number of patients, due to both the rarity of the condition described and the inevitable drawbacks in patients’ recruitment, comprise the major limitations of our study.
Non-invasive evaluation of myocardial ischemia by myocardial perfusion imaging does not seem to be of interest in this setting [15-17].DSCT could be the preferred initial testing modality in patients with severely dilated PA as assessed by TTE for LMCS exclusion. Magnetic resonance imaging with gadolinium (a non-iodine based contrast) might also be used for patients with iodine or shellfish allergies, or for patients with renal dysfunction but is not permitted in the presence of devices or other metallic implants and is less cost-effective. DSCT is safe and seems to provide useful information regarding LMCS presence. Two patients in our study had LMCS according to DSCT, verified with CCA. CCA in conjuction with IVUS is described to be necessary for diagnosis.
In case of LMCS detection, it is crucial to restore unobstructed coronary flow. Treatment is indicated when angiographic compression is documented, whereas the optimal therapeutic approach is debatable. Surgical correction of the dilated PA has been reported [14], but coronary revascularization is the procedure of choice [6]. Percutaneous coronary intervention combined with stent implantation seems to be a safe and effective option, avoiding the postoperative risk of RV failure in patients with increased PA pressure [10]. Treatment of the underlying condition resulting in LMCS is indicated as pulmonary endarterectomy in chronic thromboembolic PH [18], or atrial septal defect repair [1, 2, 19], leading to less LMCA compression due to lower pressures in PA trunk. Lung transplantation seems to be the definitive treatment option for PH patients in severe clinical condition being on PAH specific drug combination therapy [3, 8, 20, 21].
CONCLUSION
According to our study, LMCS exclusion seems to be necessary not only in patients suffering from angina. LMCS did not occur at a distance smaller than 0.50 mm between the PA and the LMCA. Moreover, we confirmed that PA over 40.00 mm is clearly associated with PH and LMCS. Finally, it seems that LMCS is not correlated with the distance between the PA and the aorta or with CI and NT-proBNP; however, it can associate the duration of disease and PASP.
We claim that a periodical rule-out of this rare entity, as assessed by DSCT, in patients with a severely dilated PA seems to be mandatory for PH patients contributing to survival improvement. The limited number of our patients, due to the rarity of the condition described, and despite the statistical adjustments for small specimens, compels us to emphasize the need for further studies, with larger series of patients suffering from LMCS detected by DSCT.
LIST OF ABBREVIATIONS
Conflict of Interest
The authors confirm that this article content has no conflict of interest.
Acknowledgements
Declared none.


