All published articles of this journal are available on ScienceDirect.
Heparin Versus Bivalirudin in Acute Myocardial Infarction: Unfractionated Heparin Monotherapy Elevated to Primary Treatment in Contemporary Percutaneous Coronary Intervention
Abstract
Bivalirudin, a direct thrombin inhibitor, was developed as an antithrombin agent for patients undergoing percutaneous coronary interventions (PCI) with the hypothesis that it would reduce bleeding complications without compromising the rate of ischemic events compared to heparin plus GP IIb/IIIa inhibitors. Although the cumulative evidence makes a strong argument for the use of bivalirudin rather than heparin plus systematic GP IIb/IIIa inhibitors for the great majority of patients with acute myocardial infarction (AMI) undergoing PCI, the benefit observed with bivalirudin was achieved because of the major bleeding complications with the use of heparin plus GP IIb/IIIa inhibitors. When bivalirudin was compared with unfractionated heparin alone there was no benefit in ischemic complications with a decrease in major bleeding. However, in a recent large randomized controlled trial comparing bivalirudin with unfractionated heparin alone in AMI patients undergoing primary PCI, bivalirudin did not reduce bleeding complications and was associated with higher rates of stent thrombosis, myocardial reinfarction, and repeat revascularization compared with heparin. Moreover, a very recent meta-analysis shed more insights on the utilization of bivalirudin versus heparin regimens during PCI. Findings from this meta-analysis suggest that routine use of bivalirudin offers little advantage over heparin among PCI patients. In a detailed analysis of some randomized trials and observational studies with bivalirudin in AMI patients done by myself and published almost five years ago in this journal, I rendered some reflections on the future widespread use of bivalirudin. “In the setting of PCI in AMI patients, and in the absence of GP IIb/IIIa inhibitors, bivalirudin did not offer any beneficial effect in the incidence of the composite end points when compared with heparin alone. For now, in real world practice, one would probably choose a well known cheaper drug that has already passed the test of time, heparin. There may be reinforcement in the sole utilization of heparin confining GP IIb/IIIa inhibitors and other intravenous antithrombotics to bailout therapy for periprocedural PCI complications in AMI patients”. Therefore, instead of being the beginning of a new era with bivalirudin, it sure is a welcome back to an old friend, heparin. Indeed, after more than two decades, it is always good to welcome back an old friend, unfractionated heparin, as monotherapy and preferred anticoagulant regimen for contemporary PCI in AMI patients.
INTRODUCTION
Under normal physiologic conditions, platelets are essencially inert; their adhesion to the subendothelial matrix is prevented by an intact vascular wall. The main role of platelets is to coordinate with vascular endothelium and soluble plasma factors in the haemostatic process [1-5]. A typical episode of acute myocardial infarction (AMI) begins with disruption of an atheroma. Plaque rupture occurs when the fibrous cap of the atherosclerotic plaque is disrupted by physical stresses and either tears or is removed completely [6-8]. When the endothelial intima is interrumpted, substances such as collagen, von Willebrand factor, fibronectin or vitronectin are exposed to circulating platelets and plasma coagulation factors [9-12]. Then, platelets spontaneously adhere to newly exposed adhesive proteins forming a protective monolayer of cells. These platelets are activated very rapidly by agonists such as thrombin, collagen, adenosine diphosphate, and thromboxane A2, causing them morphologic changes and release of materials from stored vesicles for further activation of more platelets and the propagation of the haemostatic process [9-12].
Regardless of the stimulus for platelet activation, GP IIb/IIIa antagonists inhibit thrombosis by preventing fibrinogen from binding to the platelet GP IIb/IIIa receptor, the final common pathway of platelet aggregation and subsequent thrombus formation [6-8]. However, the utilization of platelet GP IIb/IIIa inhibitors have been associated with thrombocytopenia, high rates of bleeding, and the need for transfusions, which increase costs, length of hospital stay, and mortality [13-15]. On the other hand, bivalirudin, a semi-synthetic direct thrombin inhibitor, has been shown to provide similar efficacy with less bleeding compared with unfractionated heparin plus platelet GP IIb/IIIa inhibitors in patients treated by percutaneous coronary interventions (PCI) [16-20].
The main goal in PCI is the combined utilization of stents and pharmacologic agents to quickly open both the artery and the microvasculature, and the use of adjunctive interventions to further improve flow and keep arteries open. The use of more potent antithrombin and antiplatelet agents during PCI has clearly reduced the rate of ischemic complications with an increase in the rate of bleedings [21-24]. Therefore, investigational studies have focused in pharmacological agents that would reduce bleeding complications without compromising the rate of major adverse cardiovascular events. In accord with this goal, the results of randomized trials and observational studies make a strong argument for the use of bivalirudin rather than heparin plus GP IIb/IIIa inhibitors for the great majority of patients [25-37]. However, some controversial results and limitations in the studies with bivalirudin [38-41], as well as, the observations of a recent randomized trial [36] and a very recent meta-analysis [39] exert some doubts in the future widespread use of this drug. As I stated almost five years ago in this journal [38] “rather than the beginning of a new era with bivalirudin, this may be the beginning of a more rational utilization of GP IIb/IIIa inhibitors, and other antithrombotic drugs, and reinforcement in the sole utilization of unfractionated heparin in the treatment of AMI patients with the PCI strategy” [38].
COMPARISON OF BIVALIRUDIN WITH UNFRACTIONATED HEPARIN IN ACUTE MYOCARDIAL INFARCTION
Bivalirudin, a direct thrombin inhibitor, was developed as an antithrombin agent for patients undergoing PCI with the hypothesis that it would reduce bleeding complications without compromising the rate of ischemic events compared to heparin plus GP IIb/IIIa inhibitors. The Harmonizing Outcomes with revascularization and stents in ST Elevation Myocardial Infarction (HORIZON STEMI) trial studied patients whom underwent primary PCI in AMI [18]. In this trial, bivalirudin compared with unfractionated heparin plus platelet GP IIb/IIIa inhibitors resulted in less major bleeding (4.9% vs. 8.3%, P<0.001), and similar rate of major adverse cardiovascular events at 30 days (5.4% vs. 5.5%, P=0.95). The primary endpoint, a composite of major adverse cardiovascular events or major bleeding (net adverse clinical events), was significantly less with bivalirudin (9.2% vs. 12.1%, P=0.005). Furthermore, 30-day mortality was significantly less with bivalirudin (2.1% vs. 3.1%, P=0.047), and there was less thrombocytopenia (0.5% vs. 1.1%, P=0.02). The frequency of stent thrombosis in the first 24 hr after PCI was greater in the bivalirudin group (1.3% vs. 0.3%, P <0.001). However, at 30 days, there were no significant differences between groups (2.5% vs. 1.9%, P=0.30). Similar results were observed by the EUROMAX study [34] which also had an open-label design and assessed antithrombin treatment started during transport, comparing bivalirudin against either unfractionated or low molecular weight heparin. The HORIZONS STEMI study was the first trial to show a significant reduction in mortality with any adjunctive therapy used with primary PCI in AMI patients. However, since mortality was not a primary or secondary end point, this result could have occurred just by chance and should be taken with caution. In addition, the study was not powered to detect differences in mortality between both groups. Nevertheless, there are certain factors that could explain this mortality difference and major bleeding is one of them. In patients with acute coronary syndromes whom underwent PCI procedures, major bleeding complications have been shown to be strong predictors of subsequent stent thrombosis, other ischemic events, and death [42, 43]. Moreover, major bleeding has been shown to be the strongest predictor of 30-day mortality in PCI treated patients with acute coronary syndromes, even stronger than periprocedural myocardial infarction [42, 43]. Major bleeding can contribute to mortality due to mortality from the bleeding event itself, the need for transfusions, and the need for discontinuation of antiplatelet and anticoagulant therapy which may predispose to stent thrombosis and other ischemic events. Blood transfusions are associated to significantly increased morbidity and mortality, increased infections, and increased hospital stay and costs [44, 45]. It is very clear that in the HORIZON trial, the benefit observed with bivalirudin was achieved because of the major bleeding complications with heparin plus GP IIb/IIIa inhibitors. This fact is more notorious when it is compared to other studies that leave GP IIb/IIIa inhibitors out of the scenario (Figs. 1 and 2). In this regard, Bonello L et al. [46] evaluated the safety and efficacy of bivalirudin versus heparin when used without GP IIb/IIIa inhibitors during primary PCI in AMI patients. They observed that the rate of major bleeding was identical in the two groups (4.1% vs. 4.2%, P=0.92). Although there was a trend toward less hematoma in the bivalirudin group, it did not reach statistical significance (3.4% vs. 6.7%, P=0.09). The rate of major hematoma was identical between the two groups as well (0.5% vs. 0.6%, P=1). There was no difference in the rates of death (1.1% vs. 0.9%, P=1), or major adverse cardiovascular events (2.7% vs. 1.2%, P=0.15) in the bivalirudin and heparin groups, respectively. No stent thrombosis occurred during in-hospital stay. Therefore, the results of their study suggest that in primary PCI for AMI, bivalirudin versus heparin when used without GP IIb/IIIa inhibitors exhibits similar results in terms of ischemic and bleeding complications.
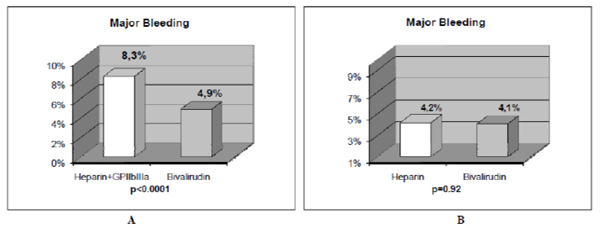
The results of major bleeding in 2 studies with AMI patients treated with primary PCI are shown. The comparison of bivalirudin to heparin plus GP IIb/IIIa inhibitors in the HORIZONS trial [18] (Fig. 1A), and, to heparin alone in the study of Bonello L et al. [46], (Fig. 1B) is depicted. When bivalirudin is compared with heparin, there is only a significant difference in major bleeding in AMI patients undergoing PCI only when GP IIb/IIIa inhibitors are systematically added to unfractionated heparin, but not when bivalirudin is compared to heparin alone without the use of GP IIb/IIIa inhibitors. Reprinted with permission from Centurión OA et al. Actual role of platelet glycoprotein IIb/IIIa receptor inhibitors as adjuntive pharmacological therapy to primary angioplasty in acute myocardial infarction: In the light of recent randomized trials and observational studies with bivalirudin [38].
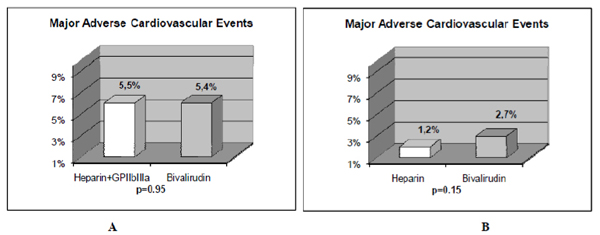
The results of major adverse cardiovascular events in 2 studies with AMI patients treated with primary PCI are shown. The comparison of bivalirudin to heparin plus GP IIb/IIIa inhibitors in the HORIZONS trial (Fig. 2A) [18], and, to heparin alone in the study of Bonello L et al. [46] (Fig. 2B) is depicted. There was no significant difference in major adverse cardiovascular events in AMI patients undergoing PCI when bivalirudin was compared with unfractionated heparin with or without the use of GP IIb/IIIa inhibitors. Reprinted with permission from Centurión OA et al. Actual role of platelet glycoprotein IIb/IIIa receptor inhibitors as adjuntive pharmacological therapy to primary angioplasty in acute myocardial infarction: In the light of recent randomized trials and observational studies with bivalirudin [38].
There is only one large randomized controlled trial comparing bivalirudin with unfractionated heparin alone in AMI patients undergoing primary PCI. The HEAT-PPCI trial [36] was an open-label, single center, randomized controlled study that enrolled 1812 patients undergoing emergency coronary angiography in the setting of acute myocardial infarction. It was found that the use of heparin, rather than bivalirudin, confers significant advantage in the avoidance of major adverse events. In the HEAT-PPCI trial [36] bivalirudin did not reduce bleeding complications and was associated with higher rates of stent thrombosis, myocardial reinfarction, and repeat revascularization compared with heparin (Figs. 3 and 4). These results are consistent with the finding of a statistical significant increase in acute stent thrombosis, associated with bivalirudin therapy, in both HORIZONS-AMI trial (1.3% in the bivalirudin group vs. 0.3% in the heparin group; p<0.001) [18] and EUROMAX trial (1.1% vs. 0.2%; p=0.007) [34]. The absolute stent thrombosis rate was higher in the HEAT-PPCI trial than it was in HORIZONS-AMI, probably because of the high-risk population (28 day mortality 4.7% in the HEAT-PPCI vs. 2.6% in HORIZONS-AMI). The magnitude of the difference might also be because patients given bivalirudin were not protected by additional administration of heparin, which was reported in 65% of HORIZONS-AMI patients randomised to bivalirudin. In this latter trial there was a 1% absolute excess of 24-hour stent thrombosis with bivalirudin, and in a very recent meta-analysis of randomized trials of bivalirudin versus heparin regimens during PCI performed by Cavender MA, and Sabatine MS, it was also found an increase in the risk of stent thrombosis and myocardial infarction with bivalirudin [39]. This meta-analisys included 16 trials of nearly 34000 patients with AMI, with non-ST-segment elevation ACS, or urgent or elective PCI [39]. Regarding ischaemic complications of the interventional procedure, they were more frequent among patients assigned to receive bivalirudin-based regimens compared with heparin-based regimens (risk ratio [RR] 1.09, 95% CI 1.01-1.17; p=0.02), a finding that was consistent regardless of the clinical indication for PCI or the strategy for the utilization of GP IIb/IIIa inhibitors [39]. However, considering bleeding complications, the effect of bivalirudin on bleeding risk was significantly affected by the strategy for concomitant GP IIb/IIIa inhibitors utilization. In those trials in which GP IIb/IIIa inhibitors were used systematically with heparin, bleeding was reduced by bivalirudin (RR 0.53, 95% CI 0.47–0.61). One of the significant and very important finding of this meta-analysis is that in those trials which compared bivalirudin with heparin monotherapy, that is, leaving GP IIb/IIIa inhibitors out of the scenario, there was no significant difference in bleeding rates (RR 0.78, 95% CI 0.51-1.19), but an increase in ischemic complications with the use of bivalirudin [39]. The ongoing multicenter MATRIX trial [47], which will be completed by the end of 2014, will certainly shed further light on this manner.
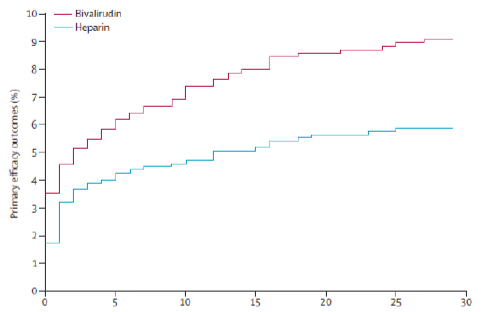
Event curve for primary efficacy outcome (combination of death, cerebrovascular accident, reinfarction, and unplanned target lesion revascularisation), censored for the first event. Reprinted with permission from Shahzad A, Kemp I, Mars C, Wilson K, Roome C, Cooper R, et al. Unfractionated heparin versus bivalirudin in primary percutaneous coronary intervention (HEAT-PPCI): an open-label, single centre, randomized controlled trial [36].
There was only one trial [16] analized in the recent meta-analysis [39] that found a reduced hemorrhage risk with bivalirudin, because they used a heparin dose (140 U/kg bodyweight) that was much higher than that used in interventional practice (50-70 U/kg). Findings from this meta-analysis [39] suggest that routine use of bivalirudin offers no advantage over heparin among patients undergoing PCI. Moreover, rates of myocardial infarction, revascularization, and stent thrombosis were reduced by heparin versus bivalirudin, independently of concomitant utilization of GP IIb/IIIa inhibitors. Therefore, these findings provide a rationale to consider heparin monotherapy as the preferred anticoagulant regimen for contemporary PCI. In this regard, in a detailed analysis of some randomized trials and observational studies with bivalirudin in AMI patients done by myself and published almost five years ago in this journal [38], I rendered some reflections on the future widespread use of bivalirudin. “In the setting of PCI in AMI patients, and in the absence of GP IIb/IIIa inhibitors, bivalirudin did not offer any beneficial effect in the incidence of the composite end points when compared with heparin. For now, in real world practice, one would probably choose a well known cheaper drug that has already passed the test of time, heparin. There may be reinforcement in the sole utilization of heparin confining GP IIb/IIIa inhibitors and other intravenous antithrombotics to bailout therapy for periprocedural PCI complications in AMI patients” [38]. Therefore, in the setting of contemporary PCI in AMI patients, instead of being the beginning of a new era with bivalirudin it is a welcome back to an old friend, heparin. Indeed, after more than two decades, it is always good to welcome back an old friend, unfractionated heparin, as monotherapy and preferred anticoagulant regimen for contemporary PCI in AMI patients.

Event curve for primary safety outcome (major bleeding), censored for the first event. Reprinted with permission from Shahzad A, et al. Unfractionated heparin versus bivalirudin in primary percutaneous coronary intervention (HEAT-PPCI): an open-label, single centre, randomized controlled trial. Published online Lancet July 5, 2014 http://dx.doi.org/ 10.1016/S0140-6736(14)60924-7.
CONCLUSION
In the light of recent studies done in contemporary PCI utilization, it was demonstrated that, in the absence of GP IIb/IIIa inhibitors, bivalirudin did not offer any beneficial effect in the incidence of the composite end points when compared to unfractionated heparin. Therefore, the results of the studies available until now exert some doubts in the future widespread use of bivalirudin. For now, in the real world practice, one would probably choose a well known cheaper drug that has already passed the test of time, unfractionated heparin. With current findings in ACS patients undergoing contemporary PCI, it seems unlikely from now on that there will be an increase in the utilization of bivalirudin, instead there may be a reinforcement in the sole utilization of unfractionated heparin confining GP IIb/IIIa inhibitors and other intravenous antithrombotics to bailout therapy for periprocedural PCI complications. Therefore, these findings provide a rationale to consider heparin monotherapy as the preferred anticoagulant regimen for contemporary PCI. After more than two decades, it is always good to welcome back an old friend, unfractionated heparin, as monotherapy and preferred anticoagulant regimen for contemporary PCI in AMI patients. Indeed, heparin is the pharmacological agent to beat for future randomized trials of new drugs during contemporary PCI.
CONFLICT OF INTEREST
The author confirms that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.


