All published articles of this journal are available on ScienceDirect.
Tricuspid Valve Regurgitation Following Temporary or Permanent Endocardial Lead Insertion, and the Impact of Cardiac Resynchronization Therapy
Abstract
Background:
While some studies indicate that permanent pacemaker implantation is associated with development of tricuspid regurgitation (TR), other studies indicate no association.Little is known about the impact of temporary lead insertion during ablation procedures, or whether therapy (CRT) prevents TR post-device implantation.
Hypothesis:
We hypothesized that permanent, but nottemporary endocardial leads, are associated with development of TR, and that CRT would prevent (physiologic) TR.
Methods:
We performed a retrospective study of consecutive patients who underwent first device or radiofrequency catheter ablation over a 12-month period at a single, tertiary academic center who underwent pre- and post-procedure echocardiography.
Results:
In the 89 patients in the device group, the degree of TR significantly increased ≥ 1 grade post-permanent lead implantation: 9 had less TR, 46 were unchanged, and 34 had more TR(p=0.005). TR increased in the 62 patients who underwent device implantation without CRT (p=0.005), but did not increase in the 27 patients with CRT (p=0.47). In the 66 patients in the ablation group, there was no significant change in TR post-ablation: 8 had less TR, 48 were unchanged, and 10 had more TR (p=0.31).
Conclusion:
Permanent endocardial lead implantation was associated with an increase in TR; however, patients who underwent device implantation with CRT did not have an increase in TR.Temporary lead insertion during ablation was not associated with changes in the degree of TR. A large, prospective study is needed to accurately define the incidence and exact mechanisms of permanent endocardial lead-related TR.
INTRODUCTION
Over the last decade, there has been a significant increase in the number of cardiac device implantations –permanent pacemakers (PPM), implantable cardiac defibrillators (ICD) and biventricular pacemakers (BiV)− worldwide [1-3]. While some small, mainly retrospective studies, case reports and reviews have suggested that permanent endocardial lead implantation can lead to tricuspid regurgitation (TR) [4-13], other studies have not demonstrated any association between device implantation and the development of (TR) [14, 15]. In addition, the impact of cardiac resynchronization therapy (CRT) on the development of TR post-device implantation has not been defined. Furthermore, patients undergoing radiofrequency catheter ablation for atrial and ventricular arrhythmias routinely undergo temporary pacemaker lead insertion, often using multiple leads that cross the tricuspid valve [16,17]. Yet, very little is known about the effects of temporary lead insertion on TR severityin these patients [18], and no studies have assessed the effects of both temporary and permanent lead insertion on tricuspid valve function. In our study, we hypothesized that patients undergoing temporary lead insertion would not have a significant increase in the degree of TR post-procedure, whereas patients undergoing permanent lead insertion via device implantation would have a significant increase in the severity of TR. In addition, we hypothesized that CRT would prevent the development of (physiologic) TR in patients undergoing permanent device implantation.
METHODS
Patient Population
We performed a retrospective study of consecutive patients who underwent first device implantation (PPM, ICD, BiV), or catheter ablation, between August 2011 and August 2012 at a single, tertiary,academic medical center. Patients were included if a two-dimensional (2D) trans-thoracic echocardiogram was performed pre-procedure and within 12 months following device implantation or catheter ablation. We excluded patients under the age of 18, patients who had prior ablation or device implantation, and patients with congenital heart disease, as many of the latter patients have other structural cardiac reasons for TR. The study was approved by our institutional Research Ethics Board.
Data Collection
The medical records of all patients undergoing catheter ablation or device implantation during the study period were reviewed for the study inclusion and exclusion criteria. Clinical variables analyzed included age, sex, hypertension, diabetes, smoking status, dyslipidemia, significant coronary artery disease (>/=50% stenosis of any major epicardial coronary at angiography or a positive functional study demonstrating ischemia), clinical heart failure, chronic kidney disease (estimated GFR <60 ml/min), and baseline medication use. Echocardiographic variables included the degree of TR, RV size and function, right atrial size, pulmonary artery systolic pressure (PASP), left ventricular ejection fraction, left atrial size, aortic valve stenosis or regurgitation and mitral valve stenosis or regurgitation [19, 20]. The degree of TR was based on the following ordinal scale: none/trivial, mild, moderate and severe [20]. A change in TR was defined as a change of at least one grade (for example, from moderate to severe or moderate to mild). RV size and function, LA size and RA size were also assessed using an ordinal scale; normal, mild, moderate and severe.
Statistical Analysis
Baseline patient characteristics were compared between the device and ablation group using the Student’s t test for continuous variables, and the Fisher’s exact test for categorical variables. The difference between ordinal echocardiographic variables (ie. pre- and post procedure TR) was tested using the McNemar-Bowker test.
RESULTS
A total of 1041 patients underwent first device implantation or catheter ablation between August 2011 and August 2012. Of these, 527 patients underwent device implantation, and 524 patients underwent radiofrequency catheter ablation. In total, 155 patients met study inclusion criteria, and were therefore analyzed. Of the 89/155 (57%) patients who underwent permanent device implantation, 37 patients received an ICD, 25 patients received a BiV-ICD, 26 patients received a PPM and one patient received a BiValone. The remaining 66/155 (43%) patients underwent radiofrequency catheter ablation for arrhythmia; 28 patients underwent atrial fibrillation ablation, 21 patients underwent atrioventricular re-entry ablation, 15 patients underwent atrial flutter ablation, and 2 patients underwent ventricular tachycardia ablation.
The patient characteristics of the device and ablation groups are shown in (Table 1). As expected, patients undergoing device implantation had more cardiovascular risk factors, coronary artery disease, and congestive heart failure, compared to the ablation group (Table 1).The mean time to follow up echocardiographic study was 5.6 months (SD ± 3.9) in the device group and 4.7 (SD ± 3.6) months in the ablation group (p=0.39 for comparison).
In patients undergoing permanent device implantation, the degree of TR increased in 34 patients, decreased in 9 patients, and was unchanged in 46 patients (p=0.005 for comparison) (Table 2, Fig. 1).While the 42 patients who underwent device implantation without CRT had a significant increase in TR (p=0.005), the 27 patients who underwent device implantation with CRT had no significant increase in TR (p=0.47) (Table 3). In the device group, there was no significant difference in right ventricular size or systolic function (p=0.68 and p=0.42 respectively), left ventricular ejection fraction (37.9%±3.7vs. 37.5%±4.5, p=0.82) or left sided valve disease observed (Table 4).
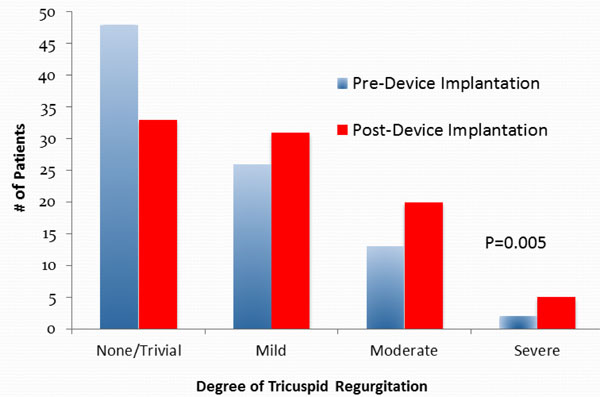
Tricuspid Valve Regurgitation Pre- and Post-Permanent Device Implantation. There was a significant increase in the degree of tricuspid regurgitation from pre- to post-device (permanent pacemaker, implantable cardiac defibrillator or biventricular pacemaker] insertion (p=0.005).

Tricuspid Valve Regurgitation Pre- and Post-Radiofrequency Catheter Ablation Procedure. There was no significant change in the degree of tricuspid regurgitation from pre- to post-radiofrequency catheter ablation (p=0.30).
Baseline characteristics of patients undergoing first device implantation or catheter ablation.
| Patient Characteristics | Device group N=89 (%) | Ablation group N=66 (%) | P-value |
|---|---|---|---|
| Mean Age, years (SD) | 69.6 (13.1) | 58.1 (16.5) | <0.001 |
| Female | 58 (65.2) | 35 (53.0) | 0.273 |
| Diabetes Mellitus | 17 (19.1) | 12 (18.2) | 0.888 |
| Hypertension | 64 (71.6) | 24 (36.4) | <0.001 |
| Any Smoking History | 45 (50.1) | 15 (22.7) | <0.001 |
| Dyslipidemia | 62 (69.7) | 23 (34.3) | <0.001 |
| Coronary Artery Disease | 54 (60.7) | 8 (12.1) | <0.001 |
| Clinical Heart Failure | 42 (47.2) | 1(1.5) | <0.001 |
| *Chronic Kidney Disease | 22 (23.6) | 3 (4.5) | 0.001 |
| Medications | |||
| Aspirin | 41 (46.1) | 20 (30.3) | 0.047 |
| Oral Anticoagulant | 38 (42.7) | 36 (54.5) | 0.144 |
| Beta Blockers | 77 (86.5) | 33 (50) | <0.001 |
| Calcium Channel Blockers | 10 (11.2) | 28 (42.4) | <0.001 |
| Digoxin | 12 (13.5) | 3 (4.5) | 0.063 |
| Class III Anti-arrhythmic | 12 (13.5) | 16 (24.2) | 0.085 |
| ACE-inhibitors or Angiotensin Receptor Blockers | 67 (75.3) | 22 (33.3) | <0.001 |
| Statins | 59 (66.3) | 21 (31.8) | <0.001 |
| Aldosterone Antagonists | 35 (39.3) | 7 (10.8) | <0.001 |
| Diuretic | 48 (53.9) | 5 (7.7) | <0.001 |
* Chronic Kidney Disease defined as glomerular filtration rate < 60 mls/min.
Tricuspid regurgitation (tr) degree before and after device implantation and ablation.
| Device (n=89) | Ablation (n=66) | |||||
|---|---|---|---|---|---|---|
| TR Degree | TR Before | TR After | p-value | TR Before | TR After | p-value |
| None/trivial | 48 (54%) | 33 (37%) | 0.005 | 50 (76%) | 48 (73%) | 0.30 |
| Mild | 26 (29%) | 31 (35%) | 13 (19%) | 11 (17%) | ||
| Moderate | 13 (15%) | 20 (22%) | 1 (2%) | 6 (9%) | ||
| Severe | 2 (2%) | 5 (6%) | 2 (3%) | 1 (2%) | ||
TR degree before and after device implantation with or without cardiac resynchronization therapy (crt).
| CRT(n=27) | No CRT(n=62) | |||||
|---|---|---|---|---|---|---|
| TR Degree | TR Before | TR After | p-value | TR Before | TR After | p-value |
| None/Trivial | 11 (41%) | 11 (41%) | 0.47 | 37 (60%) | 22 (35%) | 0.005 |
| Mild | 10 (37%) | 9 (33%) | 17 (27%) | 22 (35%) | ||
| Moderate | 6 (22%) | 6 (22%) | 7 (11%) | 14 (23%) | ||
| Severe | 0 (0%) | 1 (4%) | 2 (3%) | 4 (6%) | ||
Echocardiographic measurements at baseline and follow up.
| Variable | Device Group | Ablation Group | ||||
|---|---|---|---|---|---|---|
| Pre (%) | Post (%) | P value | Pre (%) | Post (%) | P value | |
| Right Ventricular Size | ||||||
| Normal | 79.8 | 71.9 | 0.68 | 92.4 | 97 | 0.38 |
| Mild | 11.2 | 12.4 | 6.1 | 3 | ||
| Moderate | 7.9 | 10.1 | 1.5 | 0 | ||
| Severe | 0 | 2.2 | 0 | 0 | ||
| Right Ventricular Function | ||||||
| Normal | 66.5 | 62.9 | 0.42 | 95.5 | 97 | 0.63 |
| Mild | 14.6 | 12.4 | 4.5 | 1.5 | ||
| Moderate | 12.4 | 12.4 | 0 | 1.5 | ||
| Severe | 3.4 | 7.9 | 0 | 0 | ||
| Right Atrial Size | ||||||
| Normal | 43.8 | 33.7 | 0.15 | 62.1 | 65.2 | 0.15 |
| Mild | 14.6 | 14.6 | 18.2 | 9.1 | ||
| Moderate | 15.7 | 13.5 | 6.1 | 9.1 | ||
| Severe | 5.6 | 14.6 | 6.1 | 4.5 | ||
| Pulmonary Hypertension | ||||||
| None | 49.4 | 48.3 | 0.32 | 51.5 | 56.1 | 0.71 |
| Mild | 16.9 | 18 | 7.6 | 4.5 | ||
| Moderate | 14.6 | 18 | 0 | 1.5 | ||
| Severe | 2.2 | 14.6 | 0 | 0 | ||
| Left Atrial Size | ||||||
| None | 20.2 | 13.5 | 0.20 | 45.5 | 43.9 | 0.80 |
| Mild | 27 | 24.7 | 19.7 | 19.7 | ||
| Moderate | 24.7 | 29.2 | 19.7 | 12.1 | ||
| Severe | 22.5 | 29.2 | 12.1 | 19.7 | ||
| Mitral Regurgitation | ||||||
| None | 33.7 | 31.5 | 0.81 | 74.2 | 69.7 | 0.34 |
| Mild | 34.8 | 34.8 | 18.2 | 19.7 | ||
| Moderate | 24.7 | 24.7 | 6.1 | 9.1 | ||
| Severe | 4.5 | 7.9 | 1.5 | 1.5 | ||
| Aortic Stenosis | ||||||
| None | 91 | 89.9 | 1.00 | 97 | 98.5 | 1.00 |
| Mild | 7.9 | 7.9 | 1.5 | 0 | ||
| Moderate | 0 | 1.1 | 1.5 | 1.5 | ||
| Severe | 0 | 0 | 0 | 0 | ||
| Aortic Regurgitation | ||||||
| None | 85.4 | 85.4 | 1.00 | 92.4 | 93.9 | 0.50 |
| Mild | 12.4 | 12.4 | 7.6 | 3 | ||
| Moderate | 1.1 | 1.1 | 0 | 3 | ||
| Severe | 0 | 0 | 0 | 0 | ||
In patients who underwent radiofrequency catheter ablation alone, there was no significant change in the degree of TR: TR increased in 10 patients, decreased in 8 patients, and was unchanged in 48 patients (p=0.31 for comparison). (Table 2, Fig. 2) Of note, following catheter ablation, more than 90% of patients had either trivial or mild tricuspid regurgitation. There was no significant change in RV size or RV function following catheter ablation (p=0.38 and p=0.63 respectively), nor was there a statistically significant change in left ventricular ejection fraction (59.8± 4.5 vs. 58.9± 5.3,p= 0.79) observed.
DISCUSSION
This study is the first to analyze the impact of both temporary and permanent lead insertion on TR degree in patients undergoing permanent device implantation and ablation, respectively. The findings show that patients who underwent temporary lead insertion during ablation procedures did not have a significant increase in the degree of TR, whereas patients who underwent permanent lead insertion via device implantation demonstrated a significant increase in TR. Importantly, and a novel finding, patients who underwent permanent device implantation with CRT had no significant change in TR, in contrast to patients who had a permanent device without CRT, who did have a significant increase in TR.
Previous studies—primarily case reports, small retrospective studies and reviews [6-13] have described an association between permanent endocardial lead implantation and post-procedure TR. Paniagua et al. [4] reported a higher prevalence of moderate and severe TR in patients with 374 PPM leads compared to age and sex matched controls (25% vs. 12%, odds ratio 4.75,P<0.001); however, the study did not compare the degree of TR pre- and post-procedure. Klutstein et al. [6] retrospectively analyzed 545 patients before and after PPM placement and showed an increase in TR severity by two or more grades in 18% of patients. Kim et al. [7] retrospectively studied 248 patients undergoing device implantation with pre- and post-implantation echocardiograms, findings that TR increased by at least one grade in 24.2% patients (p=0.048). A recent retrospective study in 791 patients undergoing tricuspid valve surgery over 21 years at a single center showedthat a transvenous pacemaker implanted preoperatively or ≤30 days of surgery was an independent predictor of moderate to severe TR [9]. Another study of 125 patients reported an increase in significant TR from 8.7% to 31.6% (P<0.001) following device implantation [10]. On the other hand, two small studies demonstrated no significant effect of device implantation on TR severity [14, 15]. Kucukarslan et al. found that, in 61 patients referred for PPM or ICD, there was no change in TR severity at 6 months follow-up [14]. In a small prospective study of 35 patients undergoing echocardio-graphy pre- and post-PPM or ICD, there also was no change seen in the degree of TR post-device implantation. [15].
The findings of our study indicate that permanent endocardial leads are associated with an increase in TR. While there were no differences in RV size and function in patients with permanent devices at follow-up echocardiography, and the study was not designed to adjudicate clinical endpoints such as development of heart failure, the results remain important, as it is known that chronic moderate or severe TR can lead to RV failure and possibly death, although this may take many years to develop [25-26]. Significantly, patients undergoing device implantation demonstrated that cardiac resynchronization therapy (CRT) was not associated with an increase in post procedure TR. This finding an been seen as analogous to findings that CRT may reduce mitral valve regurgitation as left ventricular resynchronization decreases ventricular remodeling and subsequent atrioventricular valve functional regurgitation,although this has only been demonstrated previously in patients with mitral regurgitation [22, 23]. While mechanical mechanisms of TR post-permanent device implantation such as leaflet perforation, leaflet impingement or mechanical interference of tricuspid leaflet coaptation are important and have been previously described and verified in 41 patients undergoing TV surgery for PPM-associated TR [5], physiologic mechanisms of TR, by dyssynchronous ventricular activation and resultant induction of leaflet malcoaptation that may be reversible with de-activation of pacing and restoration of ventricular synchrony, are also important and have been described [24]. It is possible that the CRT patients in our study had less right ventricular dyssynchrony and less resultant functional (physiologic) TR and therefore did not have an increase in TR post-BIV device, as opposed to the significant increase seen in patients in our study who underwent PPM or ICD implantation without CRT. However, these putative mechanisms of TR, and the effects of CRT, would need detailed evaluation in a prospective, adequately powered study.
Only one retrospective study published in 1996 with echocardiography prior to, and after radiofrequency catheter ablation identified no significant changes in echocardiographic findings post-ablation, mentioning that valvular regurgitation did not increase . [18] The first, however, to specifically assess TR prior to and post-radiofrequency ablation and post-permanent lead insertion performed during the same period, at the same centre and by the same operators, finding that there was no significant change in TR after ablation. The differences in TR following catheter ablation and permanent lead implantation are not likely due to chance alone. Many of the proposed mechanisms of device related TR such as chronic lead impingement/restriction, scarring of leaflets or RV pacing would be important in patients with permanent, but not temporary, endocardial leads [4-16,18]. Indeed, one retrospective study suggested that in the 48 patients who received ≥3 permanent endocardial leads, there was more TR seen than in the 48 patients who only underwent implantation of 2 permanent leads (DDD pacing), suggesting that interference of TV function by (multiple) permanent leads crossing the TV may be an important mechanism of TR [27]. However, the limited power of studies in this field, and the lack of clear delineation of the true incidence and exact mechanisms of post-device TR point to the clear need for adequately powered, prospective studies of patients undergoing device implantation, especially given the global increase in PPM, ICD and BIV devices [1-3]. If future prospective studies do indeed show that permanent device implantation is a cause of clinically important TR, the current development of leadless pacemakers would be arguably even more important.
LIMITATIONS
The patients in the device group, as expected, were older, had a higher prevalence of cardiac risk factors, and structural heart disease, compared to the ablation group. However, each patient within the ablation group had echocardiography pre- and post-procedure to assess changes in TR, and thus only changes within the ablation groups were measured. Likewise, only patients within the device group were compared pre- and post-procedure for changes in TR. In this way, ablation and device patients were not directly compared for TR changes. Not all patients screened at our institution underwent pre- and post-procedure echocardiography, and thus not all patients undergoing procedures at our institution were included in the analysis. It may be argued, then, that patients were “selectively” sent for echocardiography. However, any putative selection bias would have affected patients undergoing both catheter ablation (temporary lead placement) and device implantation (permanent lead placement). Yet, we still found that increased TR grade following device implantation but not catheter ablation.In patients undergoing relatively “early” post-procedure echocardiography (mean of 5.3 ± 3.7 months for both groups), the effects of device implantation on TR severity, and particularly RV size and function, may not yet be evident. An adequately powered, prospective study, with echocardiography pre- and post-device implantation, including longer term follow-up, is therefore needed, and would best address these issues.
STUDY IMPLICATIONS
Permanent endocardial lead implantation in patients undergoing PPM, ICD and BIV devices—as opposed to radiofrequency ablation without permanent lead implantation—is associated with a significant increase in the degree of TR. However, patients who underwent device implantation with CRT did not demonstrate an increase in TR, suggesting that physiologic mechanisms of TR from ventricular dyssynchrony may have been mitigated in the CRT group. Given the increase in implantation of permanent endocardial leads worldwide, there is a need for a large prospective study to clearly define the incidence and mechanisms of device related TR, as this may have impact on clinical outcome of patients with implantable cardiac rhythm devices.
CONFLICT OF INTEREST
The authors have no relevant conflict of interest to disclose.
AKNOWLEDGMENTS
Declared none.


