All published articles of this journal are available on ScienceDirect.
Subject Body Mass Index Affects Doppler Waveform in Celiac Artery by Duplex Ultrasound
Abstract
Objective:
The aim of this study is to evaluate the effect of body mass index (BMI) on peak systolic velocity (PSV) recording in the celiac artery (CA).
Subjects & Methods:
Forty male participants were entered prospectively into the study. The subjects were divided into two groups according to their body mass index. Group A included subjects with BMI ≤25 Kg/m2 and those in group B with BMI >25 Kg/m2. The diameter and PSV at the origin of CA of subjects in both groups were recorded while the subject positioned in supine and during expiration phase and fasted for 4 hours using duplex ultrasound. Both groups were matched for age and sex. Independent Student’s t-test was used to test if there is any statistical significance between diameter and PSV in both groups.
Results:
Group A’s, average age (year, ±SD) was 29.35±1.35 and average BMI (Kg/m2, ±SD) was 23.1±1.60. Group B’s, average age was 30±2.1 and their average BMI was 31±5.1. The average diameter (cm, ±SD) of CA in group A was 0.66±0.076 and in group B was 0.80±0.066. However, the average PSV (cm/s, ±SD) was 117±28.1 in group A and 102±12.4 in group B. Independent student t-test showed statistical significance between both groups for the diameter (p=0.005) and just reached statistical significance for PSV (p=0.049).
Conclusion:
Subjects with higher BMI showed reduced PSV due to a larger CA diameter and probably due to more fatty tissue accumulation around the CA origin.
INTRODUCTION
Celiac artery compression syndrome (CACS) is a condition in which the celiac artery (CA) and celiac ganglion are compressed from above by the median arcuate ligament, which connects the diaphragmatic crura (Fig. 1). Due to individual variations of topographic relationships of the participating structures, a spectrum exists from slight compression to complete constriction of the CA [1-12]. It is a condition with a variety of different symptoms such abdominal pain, nausea and vomiting, so it is difficult to diagnose. While sonography helps with the diagnosis in many cases [13, 14], the exact diagnostic sensitivity and specificity by ultrasound are unknown. Some authors consider a peak systolic velocity (PSV) of greater than 275cm/s in superior mesenteric artery (SMA) in fasting situation and a PSV of more than 200cm/s in CA to be considered as a stenosis of more than 70% [14-19]. Although the diagnosis of CACS has historically been established by the use of computed tomography angiography (CTA), or lateral aortography, abdominal Doppler ultrasound can be a useful non-invasive tool for diagnosis of this disorder [12].
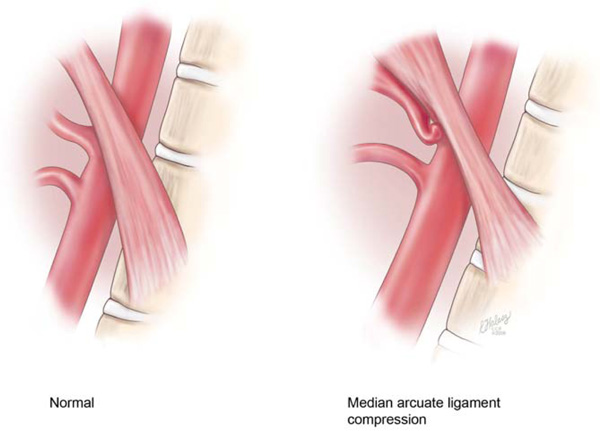
Lateral view of aorta. Left: showing normal anatomy. Right: showing median arcuate ligament compression of the celiac artery (permission from Cleveland Clinic 2012).
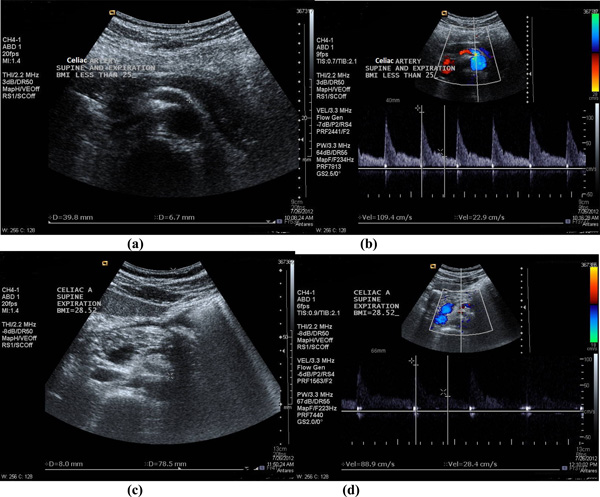
(a-b) The diameter measurement and PSV recorded in CA (note: located at 3.98cm depth from surface) in one of the subject’s in group A with BMI=20.81kg/m2, in supine position and during expiration phase [diameter =6.7mm and PSV=109.4cm/s]; and (c-d) The diameter measurement and PSV recorded in CA (note: located at 7.85cm depth from surface) in one of the subject’s in group B with BMI= 28.52 kg/m2, in supine position and during expiration phase [diameter =8mm and PSV=88.9cm/s].
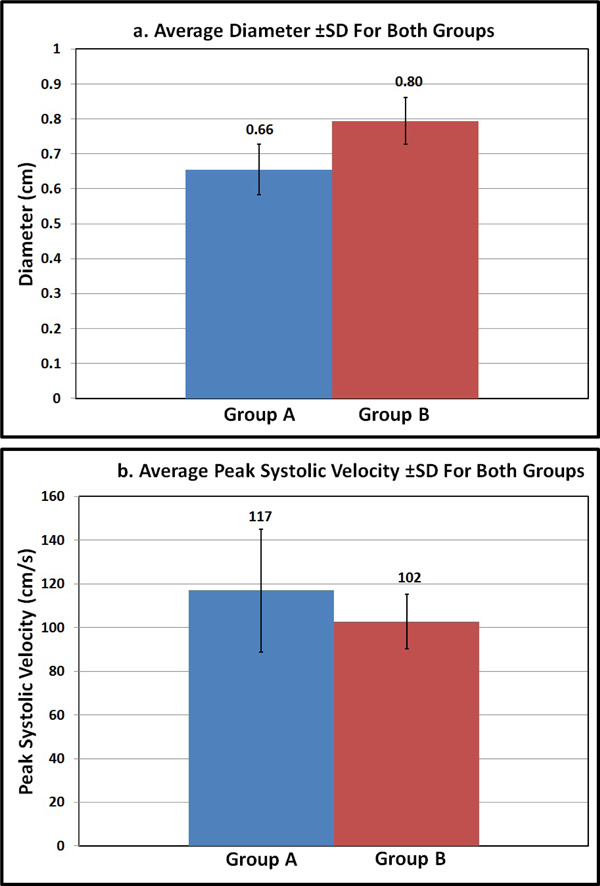
The effect of BMI on (a) Diameter (average±SD); and (b) PSV (average±SD) of the CA in both groups.
Multiple factors might affect the PSV recording in the CA causing a compression syndrome. These factors include phase of respiration [20-23] and body position during ultrasound scanning [23-26], transducer pressure over the vessel, and body mass index (BMI). In literature, there are no reports about the effect of BMI in the CA Doppler waveform especially in normal subjects. This merits prospective evaluations and prompted undertake a study to select the best technique to examine the CA during Doppler ultrasound scanning, in terms of subject BMI in normal volunteers, to help sonologists and sononographers avoid false positive diagnoses of atherosclerosis. The main aim of the present study is to assess the effect of subject BMI on the PSV in the CA in normal subjects.
SUBJECTS & METHODS
Subjects
Forty young healthy male adults were asked to participate in the present study of CA diameter and PSV measurements at the ultrasound room in Al-Sabah Hospital when they booked routinely for an upper abdomen ultrasound to rule out any pathology. They were not known to have any risk factors or any cardiovascular diseases, and their upper abdomen ultrasound was normal were included in the study. The subjects were divided into two groups according to their BMI. Group A included subjects with BMI ≤25Kg/m2 and those in group B their BMI was >25Kg/m2. Both groups were matched for age and sex. All subjects underwent duplex ultrasound with one experienced vascular sonographer who is trained in Australia. Informed consent form was obtained from all subjects.
Duplex Ultrasound Scanning Techniques
A curved 5-2 MHz transducer was used for scanning (Siemens, Antares). The subject fasted for 4-6 hours prior to the examination. The examination was performed at room temperature (22°C±2) with the subject positioned supine and during expiration. The CA was depicted in longitudinal and transverse sections while avoiding pressure. Investigation proceeded in defined steps: (1) B-mode image search for the CA for diameter measurement at its origin; (2) color Doppler overview about flow in the CA; and (3) pulsed wave Doppler for PSV measurement at its origin. Spectral display was performed at the origin of CA, always ensuring that Doppler angle is at 0° and is parallel to the vessel wall to avoid velocity recording errors. The CA diameter was recorded from the B-mode image and PSV was recorded later by placing the measurement calipers on the highest PSV of the CA Doppler waveform in the ultrasound machine.
Statistical Analysis
Data was analyzed using Statistical Package for Social Sciences (SPSS) version 19 for windows. Average age, BMI, vessel diameter, and PSV were calculated. Independent Student’s t-test was used to test if there is any statistical significance between diameter and PSV in both groups.
RESULTS
Group A, their average age (year, ±SD) 29.35±1.35 and their average BMI (Kg/m2, ±SD) was 23.1±1.60. Group B, their average age (year, ±SD) was 30±2.1, and their average BMI (Kg/m2, ±SD) was 31±5.1. Both groups were matched for age and sex. Independent Student t-test showed no statistical significance for age (p=0.25), but there was statistical significance for BMI between both groups (p=0.001).
The average diameter (cm, ±SD) of CA in group A was 0.66±0.076 and in group B was 0.80±0.066. However, the average PSV (cm/s, ±SD) was 117±28.1 in group A and 102±12.4 in group B. Independent student t-test to test for the effect of BMI on the diameter and PSV of the CA was performed and showed statistical significance for diameter (p=0.005), and just reached statistical significance for PSV (p =0.049) between both groups. The results are summarized in Table 1 and Figs. (2 and 3).
Subject Characteristics and Average±SD of the CA Diameter and PSV for Both Groups (p=0.005 for Vessel Diameter and p=0.049 for PSV)
| Group/Parameter | Group A | Group B |
|---|---|---|
| Sex (M/F) | 20/0 | 20/0 |
| Age (years) | 29.35±1.35 | 30±2.1 |
| BMI (Kg/m2) | 23.1±1.60 | 31±5.1 |
| Diameter (cm) | 0.66±0.076 | 0.80±0.066 |
| PSV (cm/s) | 117±28.1 | 102±12.4 |
In general, there was a gradual effect of BMI over CA parameter, average diameter of the CA was found to be small and the average PSV higher in group A with BMI ≤25 Kg/m2. However, group B where the BMI is >25 Kg/m2, the average diameter of the CA was larger and the average PSV was lower than in group A. PSV for all subjects was <200cm/s, which is the maximum limit of normal.
DISCUSSION
CACS is not a well-known condition with compression of the CA by the straddling median arcuate ligament. The extent of CA compression varies with varying position of the diaphragm. It is most pronounced in expiration phase of respiration while the patient in supine position [26] because of the abrupt change in direction of the CA that occurs during expiration.
Angiography is usually performed late in the evaluation of patients with CACS. It is typically preceded by multiple radiologic and endoscopic studies in order to exclude other causes of abdominal pain. Computed axial tomography and lateral aortography can confirm clinical suspicion of CACS and can also demonstrate whether ischemia can be corrected surgically [16,17]. However, duplex ultrasound imaging has been used as a non-invasive technique for anatomic and physiologic assessment of the CA [27-29]. Moneta et al. [27] compared the results of duplex ultrasound scanning with the findings on lateral aortography. They found that PSV values greater than 200cm/s defined >70% diameter-reducing stenosis of the CA. The accuracy of this cut-off value in the diagnosis of CA stenosis was 82%.
Reports focus on the effect of phase of respiration, especially expiration [20-23], and body position [23-26] as an essential factor affecting the flow velocity in the CA during duplex ultrasound scanning, whereas no reports in the literature put into consideration other factors affecting Doppler signal in the CA such as BMI. The aim of the present study is to determine the effect of BMI in Doppler velocity. This base line study will enable sonologists and sonographers alike, to have an idea about the effect of BMI in Doppler velocity waveform of the CA.
Duplex ultrasound is used in our present study because of its availability, accuracy, reproducibility, ease, costs, and avoidance of radiation [26]. Due to this one experienced sonographer who was trained in Australia performed the examination. The major aim of the one examiner in the present study is to obtain a consistent results and the measurements were not done by three examiners to evaluate the specificity and sensitivity of the sonography. However, the documentation of an increase in flow velocities at the compressed segment of the vessel in the diagnosis of CACS may have potential technical limitations and difficulties. In our groups of volunteers, acoustic access to the compressed region of the CA may be limited by gastrointestinal gas, thick anterior abdominal wall musculature, occasionally obscuring the vessel. In all our cases, constriction, angulation, and abrupt change in the direction of the CA during phase of respiration and body position could be shown with real-time imaging. However, we could not visualize the ligamentous compression of the CA along the longitudinal plane as in lateral aortography. Moreover, we are able to obtain 0° angle of incidence to allow accurate velocity calculation. Although interrogation of Doppler signals during maximum expiration, in supine body position; was more difficult in group B subjects with higher BMI, due to cephalic and posterior motion of the CA, we obtained PSV values. The PSV was higher (117±28.1, cm/s) and the diameter was smaller (0.66±0.076, cm) in group A subjects during expiration and supine body position. However, the PSV was lower (102±12.4, cm/s) and the diameter was larger (0.80±0.066, cm) in group B during expiration and while the patient scanned in supine position. We also noted that the PSV was higher in group A than group B during expiration and supine position, although still within normal limits (<200cm/s), because this groups comprised of normal volunteers. This might be due to a difference in cardiac output between both groups due to differences in BMI but we theorized this to be attributable to a small-caliber CA in group A when compared to group B, during expiration in supine body position. Also the superficial location of the CA in group A and presence of less fatty tissue around the median arcuate ligaments make the CA exposed to more compression. However, in the subjects of group B, the CA was larger in caliber, more deeply located and the median arcuate ligaments was surrounded by more fatty tissue making the CA less predisposed to compression and hence, the PSV was lower in group B than group A where the scanning was performed during the phase of expiration and in supine body position.
Downing et al. [25] concluded that among patients with elevated velocities in the CA and SMA, repeated Doppler examination with the patient standing may demonstrate normalized velocities, consistent with CACS. Depending on Downing results [25] and our previous results [26] which showed that the best subject position to examine the CA is supine position and the best phase of respiration is during inspiration, we concluded that the CA should be examined in both phases of respiration and supine and upright body positions in subjects with possible CACS. In our present study we did not repeat the scanning during inspiration and with the subject in upright position as performed in our previous study [26] because we believe that the velocities in group A to be normalized.
The results in the present study as reported only apply to healthy young males, who may not be representative of the entire population. Perhaps the results would be different in females. Also the results might be different in older individuals, where stiffer or harder due to atherosclerosis arteries may not be susceptible to compression by the median arcuate ligament. A further limitation on the study is that the sample size is small because we only included 20 subjects in each group.
In our present study, we concluded that subject BMI has an effect on the median arcuate ligament. Further studies are needed to explore the effect of gender, age, post-prandial status, patients undergoing surgical body weight reduction (gastric banding, gastric sleeve, and gastric bypass), in healthy and diseased volunteers on the Doppler waveforms in the CA. Also it would be interesting to compare the results in this study in normal subjects to patients with CACS.
CONCLUSION
Subjects with higher BMI showed reduced PSV due to a larger CA diameter and probably due to more fatty tissue accumulation around the CA origin. This makes the CA less susceptible to compression by the median arcuate ligament. BMI is an important factor while performing Doppler for patients with celiac artery disease. Further studies are needed to confirm this scientific issue.
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflicts of interest.
ACKNOWLEDGEMENT
None declared.


