All published articles of this journal are available on ScienceDirect.
Soft Tissue Attenuation Patterns Associated with Upright Acquisition SPECT Myocardial Perfusion Imaging: A Descriptive Study
Abstract
Background:
Little is known about soft tissue attenuation artifacts when an upright patient-position SPECTmyocardial perfusion imaging (MPI) system is used. In this investigation we sought to describe the patterns and frequency of attenuation artifacts associated with this type of instruments and we explored the impact of gender and body habitus on these artifacts.
Methods:
In a cross-sectional study, we described the prevalence of various soft-tissue attenuation patterns in 212 normal SPECT-MPI studies acquired with an upright patient-position imaging system.
Results:
In these 212 normal, clinically-indicated, upright-acquisition SPECT-MPIs the attenuation patterns observed were: anterior (6.1%), inferior (63.7%) and lateral (24.1%). Though uncommon, anterior attenuation trended to being more prevalent among women [9.5% vs. 3.4%, P=0.07] and was independently associated with chest circumference. Lateral attenuation was more common among women [34.7% vs. 15.4%, p=0.001] and was strongly associated with obesity (p<0.001). Inferior attenuation was more prevalent among men than women (75.2% vs. 49.5% respectively, P<0.001).
Conclusions:
Soft-tissue attenuation artifacts are common in upright-acquisition SPECT-MPI. Recognizing the frequency of these attenuation patterns and their interaction with gender and body habitus is critical for the accurate interpretation of SPECT-MPI.
INTRODUCTION
Soft tissue attenuation remains problematic for myocardial perfusion imaging (MPI) with Single Photon Emission Computed Tomography (SPECT) [1]. Supine acquisition SPECT-MPI is associated with well recognized attenuation patterns. Nonetheless, upright acquisition SPECT instruments are increasing in popularity with the emergence of mobile cardiac-SPECT cameras, commonly used in the physician’s office [2]. While attenuation correction (AC] tools are not widely used in general [3], they are even less used with upright imaging systems. Only recently, the first upright acquisition cardiac-SPECT system capable of AC has been marketed [4]. Therefore, practicing physicians are likely to continue to interpret non-corrected upright SPECT-MPI in the future as well. As with supine acquisition, the recognition of attenuation patterns associated with upright acquisition is important for accurate MPI interpretation [5]. However, attenuation patterns with upright imaging systems have not been described. We compared the attenuation patterns observed in upright vs. supine acquisition [6]. In this paper we further examined the association of the attenuation patterns seen in upright imaging with gender and body habitus.
METHODS
We conducted a retrospective cross-sectional evaluation of 459 consecutive clinically-indicated outpatient SPECT-MPI studies performed between August 2007 and July 2008 using an upright-acquisition cardiac-SPECT system MAIcam180® (Mid-Atlantic Imaging Services, Inc. - Columbia, MD), which “passed” a SPECT phantom study conformed to ACR guidelines [7]; scoring “satisfactory” for spatial resolution, uniformity and contrast. A one-day Technetium-99m tetrofosmin protocol was implemented [8]. Stress and acquisition protocols conformed to ASNC guidelines [9-11]. Image processing and perfusion analysis were performed using MIRAGE® software (Segami Corporation - Columbia, MD). Segmental wall motion analysis was performed using Cedars-Sinai® Cardiac Suite (QGS) software (Los Angeles, CA).
To isolate attenuation artifacts from perfusion abnormalities we evaluated only normal MPI studies in a population with a low prevalence of coronary artery disease (CAD). First, we excluded subjects with clinically established CAD or typical angina [12]. Second, all MPI scans were compared quantitatively to a database of normal MPI scans from individuals with a low likelihood of CAD obtained on an identical camera. All subjects with a summed stress score (SSS) > 2, summed difference score (SDS) > 0 and those with quantitatively identified wall motion abnormalities were excluded. Consequently, we were left with 212 subjects. Third, we confirmed that the remaining group of patients were at low CAD risk; as the calculated 10-year Framingham coronary heart disease risk [12] was low [12.42% (±9.2)] and the mean probability of obstructive CAD among symptomatic patients according to Diamond and Forrester prediction rules [13] was also low [16.21% (±8.1)]. By this 3-step process, we ensured that our study population had a low likelihood of CAD and quantitatively normal MPI scans. We thus had a high level of confidence that the vast majority of MPI scans included in the analysis were “truly” normal. Therefore, we considered any reduced activity identified by subsequent analysis to be due to attenuation rather than perfusion abnormality.
In the absence of a standard definition of an attenuation defect, we adopted a methodology to define them in a dichotomous fashion. Attenuation defects were classified a priori based on their locations on a 17-segment model [11] to anterior, inferior and lateral, as defined in (Fig. 1). Only stress-SPECT images were analyzed quantitatively to identify attenuation defects. The quantitative analysis was performed using a 17-segment polar map display of photon count statistics normalized in percentage to the highest count pixel. An attenuation defect was considered to be “present” if the normalized count statistics in one or more contiguous myocardial segments was decreased by more than 20 percentage points from that of the highest activity segment in the plot. Normalized count percentages in all contiguous segments constituting one defect were averaged and tabulated. The defect was assigned a classification (anterior, inferior or lateral) based on the location of the segment of minimum activity. The detailed methodology used in this study was published elsewhere [6]. The “density” of an attenuation defect was considered to be the inverse of normalized cont statistics of photon activity; as the lower the normalized cont statistics in a defect was, the denser the attenuation.

Definition of attenuation defects. A.Anterior wall attenuation was defined as reduced activity in the anterior segments with or without involvement of the anterolateral and/or anteroseptal segments. B.Inferior wall attenuation was defined as reduced activity in the inferior segments with or without involvement of the inferoseptal and/or inferolateral segments. C.Lateral wall attenuation was defined as reduced activity in the inferolateral and/or anterolateral segments, but sparing the anterior and inferior segments. Reprinted with permission (Chawla D et al. J Nucl Cardiol. 2011 (6)).
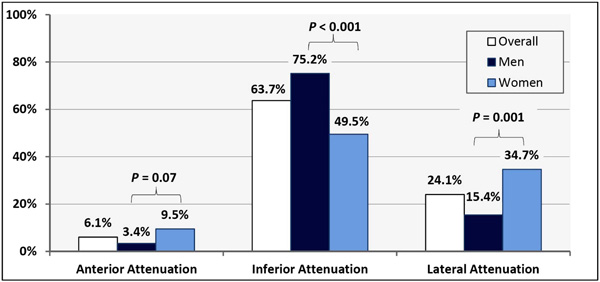
Rates of Attenuation Patters Associated with Upright Acquisition System.
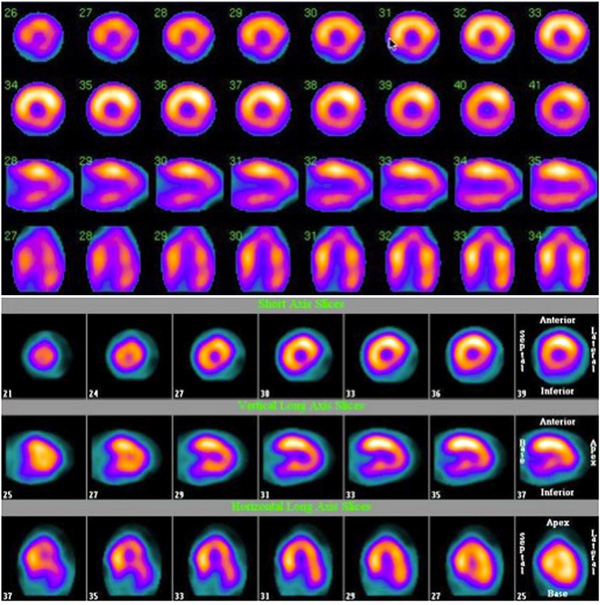
A. SPECT-MPI images depicting inferior and inferoseptal attenuation in a woman who underwent an upright image acquisition. B. SPECT-MPI images depicting inferolateral attenuation in an obese woman who underwent an upright image acquisition.
The chi-square test was used to compare the prevalence of attenuation defects between various subgroups. Multivariable logistic regression models were used to analyze the independent factors associated with various attenuation defects. The rates of observed defects were reported as proportions and odds-ratios (OR) with 95% confidence intervals (95%CI). Linear correlation between normalized count statistics in a defect, BMI and chest circumference was investigated using Pearson’s method. Only two-tailed p-values were reported. The study was internally funded and approved by the institutional review board of Rush University Medical Center.
RESULTS
We analyzed 212 subjects. The baseline characteristics of the study population are summarized in (Table 1). The prevalence of attenuation patterns observed in the entire study population and in each gender group is detailed in (Table 2, Fig. 2).
Baseline Characteristics
| Number of subjects | 212 |
| Female | 95 (44.8%) |
| Age (mean) | 55.8 (±12.5) |
| Hypertension | 64.2% |
| Diabetes Mellitus | 25.0% |
| Dyslipidemia | 46.1% |
| Smoking | 11.8% |
| Family History of CAD | 21.7% |
| Mean chest circumference (cm) | 110.0 (±11.2) |
| Male | 110.9 (±9.7) |
| Female | 109.0 (±12.7) |
| Mean BMI (Kg/m2) | 31.6 (±6.0) |
| Male | 30.1 (±5.6) |
| Female | 32.8 (±6.7) |
| Exercise Stress | 134 (63.2%) |
| CAD likelihood * | 16.21 (±8.1) |
| Framingham 10-year CHD risk | 12.42 (±9.2) |
| Mean Summed Stress Score (SSS) | 0.52 |
| Ejection Fraction (Gated SPECT) | 65.3% (±8.8) |
CAD: Coronary artery disease; BMI: Body Mass Index; CHD: coronary Heart Disease.
* Relevant to symptomatic patients only and was based on Diamond and Forrester prediction tables 12.
Rates of Attenuation Defects Observed
| Total, n=212 | Men, n=117 | Women, n=95 | Chi-square p-value (Men vs. Women) | |
|---|---|---|---|---|
| Anterior Attenuation | 13 (6.1%) | 4 (3.4%) | 9 (9.5%) | 0.07 |
| Inferior Attenuation | 135 (63.7%) | 88 (75.2%) | 47 (49.5%) | < 0.001 |
| Lateral Attenuation | 51 (24.1%) | 18 (15.4%) | 33 (34.7%) | 0.001 |
Characteristics of the Attenuation Patterns Observed
| Attenuation Pattern | Prevalence | Gender | Effect of Chest Size on Prevalence | Effect of BMI on Prevalence | Factors that Increase Severity |
|---|---|---|---|---|---|
| Anterior | Uncommon | ?F > M | + | - | Chest size |
| Inferior | Very common | M > F | - | - | BMI Chest size |
| Lateral | Common | F > M | - | + | None |
Anterior attenuation was uncommon and was observed in only 13 (6.1%) patients and trended to be less common among men; it was seen in 4 men (3.4%) vs. 9 women (9.5%) [p= 0.07, Fisher’s exact p= 0.09] (Table 2, Fig. 2). Subjects with anterior attenuation had a larger chest circumference [117.6 (±9.7) cm vs. 109.5 (±11.1) cm, p = 0.03] and trended to having a higher body mass index (BMI – Kg/m2) [34.3 (±5.96) vs. 31.4 (±5.9), p= 0.09]. Multivariable logistic regression identified chest size to be an independent factor (controlling for gender and BMI) associated with anterior attenuation (OR = 1.11 per 1 cm increase in chest circumference, 95%CI= 1.004 - 1.22, p= 0.04); whereas gender and BMI were not independent predictors of anterior attenuation (p= 0.07 and 0.3, respectively), as summarized in Table 3. Additionally, there was no interaction between female gender and chest circumference, on anterior attenuation (p= 0.46). We also inspected SPECT and rotating planar images looking for shifting anterior attenuation resulting from change in breast position between the resting and stress acquisitions. None (zero) of the study subjects demonstrated this phenomenon.
There was a moderate linear correlation between the density of anterior attenuation and chest size [Pearson’s correlation coefficient R = 0.73, p = 0.02] (Table 3). In other words; the larger the chest circumference was, the “denser” the anterior attenuation. There was no correlation between anterior attenuation and BMI (p = 0.15).
Inferior attenuation (Fig. 3) was very common and was observed in 135 (63.7%) subjects and was even more prevalent among men [88 (75.2%)] vs. women [47 (49.5%)], OR= 3.0, 95%CI= 1.7 – 5.5, p< 0.001] (Table 2, Fig. 2). There was no association between inferior attenuation and BMI or chest circumference (p= 0.24 and 0.15, respectively). Furthermore, there was no differential effect of gender on these associations (interaction p-values for BMI = 0.35 and chest circumference = 0.20). Multivariable logistic regression analysis showed that male gender was the only independent factor associated with inferior attenuation, (OR= 3.7, 95%CI= 1.8 – 7.4, p<0.001); whereas BMI and chest circumference were not associated with inferior attenuation when studied in the same model (p= 0.34 and 0.07, respectively), as shown in Table 3.
There was a modest linear correlation between the density of inferior attenuation and each of BMI R = 0.30, p = 0.001 and chest circumference [R = 0.28, p = 0.004] (Table 3).
Lateral attenuation (Fig. 3) was also quite common and was observed in 51 subjects (24.1%). It had a strong univariate association with female gender: observed in 33 (34.7%) women vs. 18 (15.4%) men (OR= 2.92, 95%CI= 1.52 - 5.65), p= 0.001 (Table 2, Fig. 2). Additionally, it was strongly associated with obesity (BMI ≥ 30): observed in 36 (32.1%) obese vs. 15 (15.0%) non-obese subjects (OR= 2.7, 95%CI= 1.4 - 5.3), p= 0.004. The association between lateral attenuation and obesity among women was notable: observed in 25 of 59 (42.4%) women with BMI ≥ 30 vs. 8 of 36 (22.2%) women with BMI < 30 OR= 2.6, 95%CI= 1.01 - 6.6, p= 0.045. This association became even more significant in markedly obese women (BMI ≥ 33) [OR= 5.2, 95%CI= 2.1 - 13.0, p< 0.001]. When tested in a multivariable model, both female gender (OR= 2.6, 95%CI= 1.1 - 5.8, p-value 0.03) and BMI (OR= 1.12 per 1 unit increase in BMI, 95%CI= 1.02 - 1.24, p= 0.02) were independent predictors of lateral attenuation (Table 3). Additionally, marked obesity (BMI ≥ 33) was associated with lateral attenuation independent of gender and chest circumference (OR = 2.82, 95%CI = 1.04 - 7.58, p= 0.04), as shown in Table 3. Furthermore, a possible interaction between female gender and marked obesity (BMI ≥ 33) was found to be associated with lateral attenuation beyond that expected from either factor alone (OR= 5.57, 95%CI= 1.1 - 28.36, p= 0.04). Among men, however, there was no significant association between lateral attenuation and obesity (p= 0.14). Although chest circumference was significantly larger among those with vs. without lateral attenuation (115.5 cm vs. 108.2 cm, respectively; p<0.001); it was not an independent factor associated with lateral attenuation when evaluated in a multivariable logistic model, and the association disappeared when BMI was introduced in the model. This suggests that chest circumference is merely a confounder, and that BMI is the true independent factor associated with lateral attenuation. Moreover, there was no correlation between the density of lateral attenuation and BMI or chest conference (p = 0.6 and 0.12, respectively).
DISCUSSION
The value of SPECT-MPI for the diagnosis and risk stratification of CAD is well-established [14]. However, the full clinical potential of SPECT-MPI is often compromised due to numerous artifacts that degrade image quality and increase the risk of image misinterpretation [15]. Soft tissue attenuation is the most frequent source of artifacts in SPECT-MPI since the heart is surrounded by tissues of varying densities and attenuation coefficients (e.g., bone, diaphragm, lungs, and breast). Thus non-uniform attenuation of myocardial photon activity is often encountered in SPECT-MPI, resulting in various patterns of attenuation artifacts depending on many factors such as: body size, depth of the heart in the body, gender, and patient position. Recognizing these attenuation patterns is crucial to optimizing the diagnostic accuracy of SPECT-MPI; as misinterpretation may lead to unwarranted downstream testing, including cardiac catheterization, at an added risk and expense.
The vast majority of the cardiac MPI attenuation literature focused on attenuation defects in supine patient position SPECT-MPI acquisition systems. To our knowledge this study is the first to comprehensively describe the attenuation patterns observed with upright SPECT-MPI and explore their interaction with body habitus and gender.
We summarize our findings in three salient points: First, anterior attenuation was the least common attenuation pattern. Only larger chest circumference was independently predictive of anterior attenuation. Additionally, the density of anterior attenuation seems to correlate with chest circumference. It is somewhat surprising that anterior attenuation was only encountered in 9.5% of women; insignificantly more common than men, which suggests that upright acquisition neutralizes, to some extent, the well-described breast attenuation in supine imaging. This finding could be explained by the fact that the breasts are stretched down by gravity and thus cover the entire heart creating milder, more uniform anterior attenuation. Furthermore, upright acquisition seems to solve the problem of a shifting breast position that can occasionally produce the “appearance” of a reversible anterior perfusion defect, as the effect of gravity would ensure exact breast position in both resting and stress acquisitions. Second, inferior attenuation was very frequently observed in both genders, but it was more prevalent among men, independent of BMI or chest circumference. It occurred in almost every male and in 1 out of 2 females. We could explain this finding by increased intra-abdominal pressure when sitting, which pushes the diaphragm up, increasing its attenuation. Additionally, the amelioration of anterior attenuation from the breast, when sitting, likely contributed to the domination of inferior attenuation in many women. Among men and women, inferior attenuation was seen independent of BMI and chest circumference. However, the density of inferior attenuation seems to modestly correlate with BMI and chest circumference. Third, lateral attenuation was commonly observed and was strongly and independently associated with female gender and obesity, but not with chest circumference. We explain this finding by possible attenuation from thick folds of skin and soft tissue covering the lateral chest wall in short, obese women when they are imaged in the sitting position with arms situated forward. Among men, lateral attenuation was not affected by obesity.
Our study has certain limitations. We acknowledge the limitation of the retrospective nature of our study. However, all MPI scans were prospectively analyzed for the sole purpose of this study. Therefore, the retrospective design is unlikely to have affected the image interpretation aspect of the study or its major conclusions. We acknowledge, however, that the estimation of coronary heart disease risk could have been slightly affected by the methodology of retrospective data collection. Additionally, we did not have an independent reference gold-standard to confirm that all scans evaluated were definitely normal. However, we demonstrated that all the scans are quantitatively normal in a population with low CAD risk and, thus, we had high confidence that they were truly normal. Furthermore, we considered MPI scans with SSS of 1 or 2 (but SDS= 0 and normal segmental wall motion) to be normal, at a small risk of including patients with “true” fixed perfusion abnormality. However, we felt that excluding these patients is more likely to exclude patients with prominent attenuation artifacts rather than those with true perfusion abnormality given the low likelihood of CAD in the population we studied. Finally, this study may be underpowered to detect certain interactions between gender and body habitus characteristics.
CONCLUSIONS
Soft-tissue attenuation artifacts were common in upright-acquisition SPECT-MPI. Inferior attenuation occurred most frequently, affecting the vast majority of males, and 1 out of 2 females. Lateral attenuation was also common, occurring in almost 1 out of 4 patients; while anterior attenuation was uncommon. Recognizing the frequency of these attenuation patterns and relearning the appearance of a normal upright study is critical for the accurate interpretation of SPECT-MPI acquired with an upright imaging system.
CONFLICT OF INTEREST
Declared none.
ACKNOWLEDGEMENTS
Declared none.


