All published articles of this journal are available on ScienceDirect.
Pulmonary Hypertension in Elderly Patients with Diastolic Dysfunction and Preserved Ejection Fraction
Abstract
Purpose:
Patients with diastolic dysfunction may have a disproportionate degree of elevation in pulmonary pressure, particularly in the elderly. Higher pulmonary vascular resistance in the elderly patients with heart failure but preserved ejection fraction suggests that beyond the post-capillary contribution of pulmonary venous congestion, a pre-capillary component of pulmonary arterial hypertension occurs. We aim to identify if pulmonary vascular resistance in elderly patients with diastolic dysfunction is disproportionately higher than patients with systolic dysfunction independent of filling pressures.
Methods:
389 patients identified retrospectively between 2003- 2010; elderly with preserved ejection fraction, elderly with depressed ejection fraction, and primary arterial hypertension who underwent right-heart catheterization at Rush University.
Results:
No significant difference in pulmonary vascular resistance between systolic and diastolic dysfunction. The mean difference in pulmonary vascular resistance was not statistically significant at 0.40 mmHg·min/l (95% CI -3.03 to 3.83) with similar left ventricular filling pressures with mean difference of 3.38 mmHg (95% CI, -1.27 to 8.02). When adjusted for filling pressures, there remained no difference in pulmonary vascular resistance for systolic and diastolic dysfunction. The mean pulmonary vascular resistance is more elevated in systolic heart failure compared to diastolic heart failure with means 3.13 mmHg·min/l and 3.52 mmHg·min/l, respectively.
Conclusion:
There was no other association identified for secondary pulmonary hypertension other than diastolic dysfunction and chronic venous pulmonary hypertension. Our results argue against any significant arterial remodeling that would lead to disproportionate pre-capillary hypertension, and implies that treatment should focus on lowering filling pressure rather than treating the pulmonary vascular tree.
INTRODUCTION
Pulmonary hypertension (PH) is caused by increased resistance at one of several sites in the pulmonary circulation, including the heart’s left chambers and valves. The pulmonary capillary wedge pressure (PCWP) is evaluated during the classification process of PH to identify the etiology of PH [1]. PH associated with a normal PCWP is classified as pre-capillary PH, and PH associated with an elevated PCWP is classified as post-capillary PH. There are many different etiologies of pulmonary hypertension, but the most common is pulmonary venous hypertension owing to left heart disease, a type of post-capillary PH [2]. This particular form of PH can occur in the setting of elevated left sided filling pressures and preserved ejection fraction (EF) [3]. Typically, the degree of elevation in pulmonary artery pressure (PAP) is concordant with the degree of elevation in the atrial pressure [4]. PH is also reported in patients with diastolic heart failure, but its prevalence and severity are poorly defined [2].
In congestive heart failure (CHF) patients, the pulmonary vasculature may undergo reactive changes from chronic elevation of the left ventricular filling pressure, resulting in severe pulmonary hypertension with a reactive increase in pulmonary venous resistance (PVR) and transpulmonary gradient (TPG). This may reflect remodeling of the arterial wall due to abnormalities of the elastic fibers, intimal fibrosis, and medial hypertrophy [5]. In one study, CHF patients with higher pulmonary pressure had greater medial hypertrophy of their pulmonary arteries secondary to their pulmonary venous hypertension [6]. Once pulmonary vascular disease progresses, the cardiac output is reduced from RV dysfunction and decreases the venous return to the left heart and with normalization of the LV filling pressure. At the time of presentation, these patients can have normal left heart filling pressure but significantly elevated pulmonary pressure, commonly misdiagnosed as PAH [2].
Few studies have investigated the occurrence of unexplained pulmonary arterial hypertension in elderly patients. Heart failure with preserved EF is particularly common among the elderly. These patients may show a disproportionate degree of elevation in their left ventricular filling pressure (LVFP) [7]. Higher pulmonary vascular resistance in the elderly patients with heart failure but preserved ejection fraction (diastolic dysfunction) suggests that beyond the post-capillary contribution of pulmonary venous congestion, a pre-capillary component of pulmonary arterial hypertension occurs. Lam C, et al., describes the passive effect of long-standing pulmonary venous hypertension leading to pulmonary arterial remodeling and, subsequently, pulmonary arterial hypertension [8]. Up to a third of patients that develop pulmonary venous hypertension will exhibit a reactive vasoconstriction of the small pulmonary arteries, resulting in increased PVR and a disproportionate increase in pulmonary artery systolic pressure (PASP) relative to the elevation of PCWP [4]. These are the patients that have been postulated to develop medial hypertrophy and intimal hyperplasia of the pulmonary arteries. We aim to describe the cohort of elderly patients with diastolic dysfunction and the degree of both pre-capillary pulmonary hypertension and post-capillary pulmonary hypertension utilizing right heart catheterization and echocardiography.
MATERIALS AND METHODOLOGY
Chart reviews of 389 patients with pulmonary hypertension were performed between July 2003 and November 2010. All patients had received a right heart catheterization to identify the etiology of their pulmonary hypertension by a pulmonary hypertension cardiology specialist at Rush University Medical Center in Chicago, Illinois, USA. PH was evaluated according to the guidelines of the American College of Chest Physicians (ACCP) and World Health Organization (WHO). Chart review and data extraction occurred retrospectively after approval by the institutional review board of Rush University Medical Center. Using the Centricity Cardiology database and Epic electronic medical record system at Rush University Medical Center, consecutive patients given a diagnosis of congestive heart failure or pulmonary hypertension after clinical evaluation including transthoracic echocardiography and undergoing right heart catheterization were evaluated. Three groups of patients were identified for comparison; systolic dysfunction, diastolic dysfunction, and normal LV function with pulmonary arterial hypertension (PAH) (Fig. 1). Inclusion criteria were pulmonary artery systolic pressure equal to or greater than 35 for all groups and pulmonary capillary wedge pressure equal to or greater than 18 on right heart catheterization for group one and two. Group one were patients identified as heart failure with decreased ejection fraction (systolic dysfunction) with age greater than 65, ejection fraction less than 50%, PCWP greater than 18, and PASP greater than 35 on right heart catheterization. Group two were identified as heart failure with preserved ejection fraction (diastolic dysfunction) and included patients with age greater than 65, preserved ejection fraction (EF greater than 50%), elevated PVR with PCWP greater than 18, and PASP greater than 35 on right heart catheterization. Group three were patients with normal LV function and primary arterial hypertension (PAH) and included patients older than 18, PCWP less than 15, and elevated PASP greater than 35 on right heart catheterization. Group three patients were categorized using the Venice classification system [9]. Patients with EF less than 50% were included in group one and excluded from groups two and three. Patients with significant cardiac valvular disease, heart transplantation, non diagnostic heart catheterization, and congenital heart disease were excluded.
Identification of Study Groups. Recruitment and identification of the study groups for patients with pulmonary hypertension who received a right heart catheterization.

Patient Distribution and Sex Differences. Total number of patients in each study group and sex distribution.
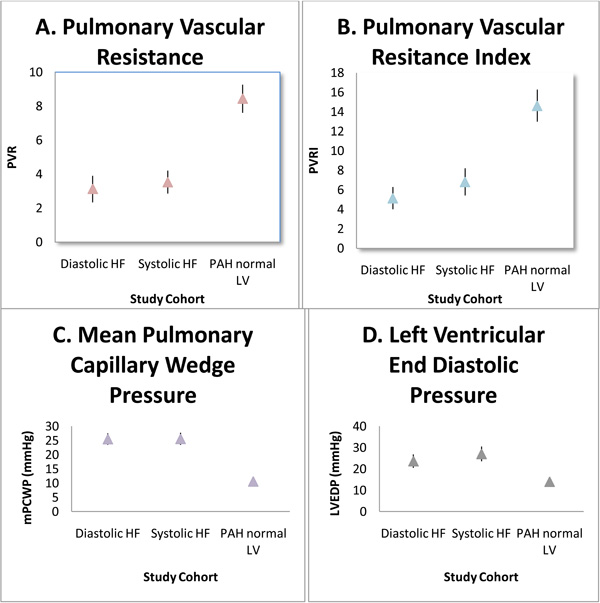
Comparison Between Groups in Hemodynamics. Comparisons between groups in hemodynamic paramaters. All values with p value <0.001. Mean values plotted (⤊) with 95% confidence interval of upper bound and lower bound.

Correlation between mPAP and mPCWP in diastolic dysfunction. Correlation between mean pulmonary arterial pressure and mean pulmonary capillary wedge pressure in diastolic dysfunction demonstrated by right-heart catheterization. Measurements are in mmHg.
Right-Heart Catheterization
Right-heart catheterization was performed by a flow-directed pulmonary artery (PA) catheter using hemodynamic and fluoroscopic guidance with oxygen saturation measurements in the wedged position. Measurements were obtained at end-expiration and averaged over three to five beats. Systemic pulse pressure (systolic – diastolic BP), pulmonary arteriolar (vascular) resistance ([mean PA pressure – mean pulmonary capillary wedge pressure (PCWP)] ÷ cardiac index [CI]), and systemic vascular resistance ([mean arterial pressure – mean right atrial (RA) pressure] ÷ CI) [indexed] were calculated and expressed Wood units ˟ meters squared. The transpulmonary pressure gradient (mean PA pressure – mean PCWP) was also calculated. Cardiac output by thermodilution and fick equation was measured.
STATISTICAL ANALYSIS
The Chi-Square test was used to compare proportions of dichotomous variables in baseline characteristics between study groups. One-way Analysis of Variance (ANOVA) was used to compare continuous variables in baseline characteristics and between study groups. Multivariate Analysis of Covariance (ANCOVA) test was used to compare PVRI, as a dependent variable, between group one and group two, adjusting for PCWP as a covariate.
RESULTS
There are a total of 389 consecutive patients collected retrospectively between the three groups from July 2003 to November 2010. All patients received a diagnostic right heart catheterization and transthoracic echocardiogram. Group one patients with systolic dysfunction account for 13.7% (n=59) of total patients. Group two with diastolic dysfunction account for 11.6% (n=50). Group three with normal left sided heart function and pulmonary arterial hypertension account for 65% (n=280) of the total patients. The majority of the patients in group three have a diagnosis of primary arterial pulmonary hypertension (n=114). Other etiologies in group three include chronic thromboembolic disease, sarcoidosis, scleroderma, and connective tissue diseases. Sex distribution and total number of patients is displayed in Fig. (2). Groups one and two are well matched. Group three has significantly more females. The average age in the elderly groups was 73 years old. As expected, group three comprised a younger population with an average age of 52 years old. Additional baseline characteristics are described in Table 1. Summary statistics for hemodynamic measurements obtained by right-heart catheterization and transthoracic echocardiogram are presented in Table 2.
Baseline Characteristics
| Diastolic Dysfunction | Systolic Dysfunction | PAH with Normal LV | P Value | |
|---|---|---|---|---|
| AGE | 74.6 | 72.4 | 52.4* | <0.001 |
| FEMALE | 31 (52.5%) | 26 (52.0%) | 192 (68.7%)* | 0.01 |
| BMI | 28.9 | 30.8 | 27.9* | 0.04 |
| RACE: White | 39 (66.1%) | 22 (44.0%) | 163 (58.2%) | |
| African American | 9 (15.3%) | 19 (38%) | 68 (24.3%) | |
| Hispanic | 2 (3.4%) | 5 (10.0%) | 19 (6.8%) | |
| Other | 9 (15.6%) | 4 (8%) | 30 (10.8%) |
* denotes statistical significant group.
Study Group Outcomes Comparing Within Groups and Between Groups
| Diastolic HF | Systolic HF | PAH Normal LV | Diastolic HF vs Systolic HF | Diastolic HF vs PAH normal LV | |||
|---|---|---|---|---|---|---|---|
| Mean Value (SD) | Mean Difference(95% CI) | P value | Mean Difference(95% CI) | P value | |||
| MAP (mmHg) | 90.1 (19) | 93.5 (23) | 93.4 (17) | 3.407 (-6.1 - 12.91) | 0.79 | 3.3 (-4.2 to 10.8) | 0.66 |
| PASP (mmHg) | 57.8 (15) | 56.8 (16) | 67.6 (26) | 1.06 (-12.6 - 10.5) | 0.99 | 9.7 (1.1 - 18.4) | 0.02 |
| mPA (mmHg) | 37.4 (10) | 38.5 (10) | * 42.6 (17) | 0.78 (-6.7 - 8.2) | 0.99 | 4.9 (-0.67 - 10.5) | 0.03 |
| mPCWP (mmHg) | 25.5 (8) | 25.5 (8) | * 10.6 (5) | 0.08 (-3.0 - 3.1) | 1.0 | 14.8 (12.5 - 17.1) | <0.001 |
| mRA (mmHg) | 15.3 (9) | 13.0 (7) | * 8.4 (6) | 2.34 (-.81 - 5.5) | 0.22 | 6.9 (4.6 - 9.2) | <0.001 |
| LVEDP (mmHg) | 23.6 (10) | 27.0 (9) | * 13.9 (6) | 3.34 (-1.3 to 8.0) | 0.24 | 9.71 (6.3 - 13.1) | <0.001 |
| CI_TD (L/min) | 2.42 (0.7) | 1.96 (.4) | 2.67 (0.9) | 0.46 (-1.5 - 0.58) | 0.66 | 0.26 (-0.17 - 1.6) | 0.67 |
| CI_Fick (L/min) | 2.15 (0.6) | 2.11 (0.7) | * 2.43 (0.8) | 0.04 (-0.35 - 0.43) | 0.99 | 0.28 (-0.17 - 1.6) | 0.17 |
| SVR (Woods Units) | 19.2 (16) | 22.6 (17) | 20.5 (14) | 3.3 (-2.4 - 9.0) | 0.43 | 1.2 (-5.8 - 3.4) | 0.90 |
| PVR (Woods Units) | 3.13 (3) | 3.52 (4) | * 8.45 (11) | 0.40 (-3.0 - 3.8) | 0.99 | 5.32 (2.8 - 7.9) | <0.001 |
| SVRI (Woods Units) | 36.9 (16) | 43.3 (17) | 37.8 (14) | 6.45 (-3.4 - 16.2) | 0.33 | 0.95 (-7.4 - 9.3) | 0.99 |
| PVRI (Woods Units) | 5.12 (3) | 6.84 (4) | * 14.6 (11) | 1.7 (-4.1 - 7.5) | 0.87 | 9.5 (5.0 - 14.0) | <0.001 |
| EF (%) | 60.5 (5) | * 30 (11) | 59.6 (10) | 30.6 (25.2 - 36.1) | <0.001 | 0.88 (-3.4 - 5.2) | 0.96 |
* denotes statistical significance with p value <0.05. MAP denotes Mean Arterial Pressure, CI confidence interval, SD standard deviation, PASP pulmonary artery systolic pressure, mPA mean pulmonary artery pressure, mPCWP mean pulmonary capillary wedge pressure, mRA mean right atrial pressure, LVEDP left ventricular end diastolic pressure, CI_TD cardiac index by thermodilution, SVR systemic vascular resistance, SVRI systemic vascular resistance index, PVR pulmonary vascular resistance, PVRI pulmonary vascular resistance index, EF ejection fraction by transthoracic echocardiogram.
There is no significant difference in PVRI between diastolic dysfunction and systolic dysfunction groups. The mean difference in PVR between diastolic dysfunction and systolic dysfunction is not significant (p-value 0.99) at 0.40 mmHg·min/L (95% CI -3.03 to 3.83) with similar left ventricular end diastolic pressures that are also not significantly different (p-value 0.24) and mean difference of 3.38 mmHg (95% CI, -1.27 to 8.02). The measures in PAP are also not significant (p-value 0.99) between diastolic dysfunction and systolic dysfunction with mean difference of 1.06 mmHg (95% CI, -10.47 to 12.59). The mean difference in PVR between diastolic dysfunction and PAH with normal LV function is significant (p-value < 0.001) at 5.32 mmHg·min/L (95% CI, 2.75 to 7.88) and LVEDP is significantly lower, as expected, with mean difference of 9.71 mmHg (95% CI, 6.32 to 13.11). PVRI is significantly higher in PAH with normal LV function compared to both heart failure groups. For clinical perspective, Fig. (3) compares the hemodynamics shown to be significant. In setting of primary arterial hypertension with significantly elevated pre-capillary pressures, it is shown that PVR and PVRI is significantly higher.
The diastolic dysfunction group did not have higher PVR than the systolic dysfunction. Furthermore, mPCWP and LVEDP is significantly higher in the heart failure groups which correlate with elevated post-capillary pressures. Nevertheless, when the elevated filling pressures measured by PCWP are adjusted for with ANCOVA analysis, diastolic dysfunction still did not show any disproportionate elevation in PVR compared to systolic dysfunction as hypothesized (p-value 0.06). In fact, the mean PVR is more elevated in systolic dysfunction compared to diastolic dysfunction with means 3.13 mmHg·min/L and 3.52 mmHg·min/L, respectively. The PAH group has significantly higher PVR than other groups with a mean of 8.45 mmHg·min/l (p < 0.001). The mPA did trend higher in the systolic dysfunction group in line with rising mPCWP as shown in Fig. (4) (p<0.001) with a standardized coefficient beta of 0.573.
DISCUSSION
Diastolic heart failure is caused by inability to produce an adequate cardiac output at normal left ventricular filling pressure despite the presence of normal systolic function [10-13]. A normal forward cardiac output can only be maintained by a compensatory elevation of left ventricular filling pressure [14]. This state is associated with a marked increase in morbidity and all-cause mortality [15]. Progression in the degree of diastolic dysfunction is associated with worsening of the prognosis of heart failure patients [16, 17]. Furthermore, in elderly patients, there is a relationship between the degree of diastolic dysfunction and pulmonary arterial hypertension [6]. Patients with pulmonary arterial hypertension can manifest with severe right ventricular hypertrophy, dilatation, and enhance ventricular interdependence and lead to elevated left ventricular diastolic pressures [7]. Bouchard et al. showed a close correlation between PASP and PCWP by echocardiography in 69 patients with normal systolic function (not all with HF) and concluded that PASP could be used as a surrogate of left ventricular filling pressure when pulmonary vascular resistance was assumed normal [18]. We also show similar results with elevation in mPA in concordance with elevations in PCWP (Fig. 4).
In patients with aortic stenosis, most of whom had a normal ejection fraction, the severity of diastolic dysfunction rather than the severity of aortic stenosis correlated best with the severity of pulmonary hypertension and a significant number of patients developed severe pulmonary hypertension [19]. Similarly, in patients with heart failure and reduced ejection fraction (systolic heart failure), it was the severity of concomitant diastolic dysfunction rather than ejection fraction or cardiac output that correlated best with the severity of pulmonary hypertension [20]. Thus, diastolic dysfunction associated with valvular disease, reduced ejection fraction, or in isolation is the common mediator that results in chronic pulmonary venous hypertension and secondary pulmonary hypertension. It is therefore not unexpected that patients with diastolic heart failure will develop pulmonary hypertension as was demonstrated in this study. In addition, we did not show any evidence of a primary pulmonary arteriopathy of late onset that may have concomitant (but unrelated) diastolic dysfunction related to their age as in previous studies [2]. Rather, the data support that there is no passive contribution of pulmonary venous hypertension to account for the increased PASP in diastolic dysfunction compared with elderly hypertensive subjects without overt heart failure that was shown by Lam et al., [8].
It seems reasonable to expect that elderly persons would be more susceptible to the development of pulmonary hypertension as age related systemic vascular stiffening has been consistently reported and age-related pulmonary artery stiffening may well occur [21-23]. This may reflect remodeling of the arterial wall due to abnormalities of the elastic fibers, intimal fibrosis, and medial hypertrophy [5]. However, the results of this study do not reveal such processes as evidenced by any disproportionate elevations in hemodynamics when compared to the systolic dysfunction group and PAH group.
It is more likely in our cohort of elderly patients, there was no etiology identified for secondary pulmonary hypertension other than diastolic dysfunction and its associated elevated filling pressures as measured by PCWP. Therefore, the increase in left ventricular free-wall stiffness led to pulmonary venous hypertension and subsequently pulmonary arterial hypertension. The degree of pulmonary arterial hypertension is not out of proportion to pulmonary venous hypertension because it was similar to the patients with systolic dysfunction and still significantly less than patients with only pulmonary arterial hypertension (Fig. 3). Neumann et al. [6] showed a significant increase in PAP for any step-up in diastolic dysfunction stage similar to what we show in the present study (Fig. 4). However, some patients have a severely elevated PA pressure with only modestly elevated left-sided filling pressure. This class of patient often causes much confusion for treating physicians because of the uncertainty of whether or not these patients would benefit or be harmed by PAH selective therapy.
In the realm of cardiac transplantation, patients have shown to have graft failure with elevated TPG. It is assumed that a TPG of <15 mmHg is acceptable for transplantation, as the elevated PA pressure is in direct proportion with the elevated left-sided filling pressure [24]. An elevated TPG is associated with a very high incidence of post-operative right ventricular failure and death. Clinical studies have shown that a high PVR is also a risk factor for graft failure due to right heart failure early after cardiac transplantation [25]. Our patients did not have significantly elevated PVR so diastolic dysfunction should be more seriously considered for cardiac transplantation.
This study is subject to the limitations of retrospective studies in which data are collected during the course of routine care. Nevertheless, the evaluation of these patients was fairly consistent. In addition this study may be susceptible to referral bias, and PCWP measurements can be erroneous. Yet, there is nothing to suggest that such errors are more frequent in elderly patients. While hemodynamic assessment of pulmonary vascular responsiveness is performed in many patients, these data are not reported because it was not consistently performed nor performed in a standardized manner. The PVR, by using the cardiac output in its equation may be unreliable because of inherent inaccuracies in the measurement of cardiac output by thermodilution, particularly at low cardiac outputs. The TPG is flow-independent and, thus, may better reflect resistance to flow across the pulmonary bed.
Age-related increases in arterial stiffening are worse in women than in men [21, 23, 26-29]. Interestingly, this study did not make the distinction in sex when evaluating hemodynamic parameters by right heart catheterization. Thus, elderly women who develop diastolic heart failure may be more prone to developing disproportionate pulmonary hypertension in response to chronic pulmonary venous hypertension associated with diastolic heart failure.
This retrospective study reveal there is no significant difference in PVR between diastolic dysfunction and systolic dysfunction groups when adjusted for PCWP. Instead the results are similar to previous studies that can show similar elevations between diastolic dysfunction and pulmonary hypertension. Our results argue against any significant arterial remodeling that would lead to disproportionate pre-capillary hypertension, and implies that treatment should focus on lowering filling pressure rather than treating the pulmonary vascular tree. In addition, patients being considered for transplantation should not be limited by their diastolic dysfunction given no increase in PVR and post-operative mortality.
CONFLICTS OF INTEREST
None


