All published articles of this journal are available on ScienceDirect.
HIV and HAART-Associated Dyslipidemia
Abstract
Effective highly active antiretroviral therapy (HAART) for human immunodeficiency virus-1 (HIV) infection has led to marked improvement in life-expectancy for those infected with HIV. Despite reductions in the incidence of AIDS with effective treatment, patients continue to experience considerable morbidity and mortality from non-AIDS illness such as premature cardiovascular disease, liver failure and renal failure. These morbidities, particularly premature cardiovascular disease, are thought to be related to a combination of the effects of an ageing HIV-infected population coupled with long-term effects of HIV infection and antiretroviral therapy (ART). One of the principle drivers behind the well documented increase in the risk of cardiovascular disease in HIV-infected patients is dyslipidemia.
This review will focus on the clinical presentation of HIV and ART-associated dyslipidemia, what is known of its patho-physiology, including associations with use of specific antiretroviral medications, and suggest screening and management strategies.
INTRODUCTION
The introduction of effective highly active antiretroviral therapy (HAART) in the mid-1990s led to a marked reduction in morbidity and mortality from human immunodeficiency virus (HIV) infection [1, 2]. Increasing life-expectancy [3], an aging population [4], and high rates of smoking [5] have led to concerns over the cardiovascular health of HIV-infected individuals in the long term. Metabolic effects of HIV infection such as hypertriglyceridemia are long recognised [6], and side effects of HAART such as dyslipidemia and insulin resistance were described very soon after its introduction [7]. Initial concerns of increased rates of myocardial infarction arising as a result of dyslipidaemia in HIV-infected patients on antiretrovirals (ARV) [8, 9, 10] have been confirmed by studies such as the D:A:D study, a large, prospective, multi-cohort study that showed associations between exposure to antiretroviral therapy and an increased risk of myocardial infarction [11]. In multivariate analyses, for every mmol/L increase in total cholesterol, the relative risk of myocardial infarction increased by a factor of 1.26 [12]. In another analysis from D:A:D which included stroke, acute myocardial infarction and invasive cardiovascular procedures there was a similar effect of hypercholesterolemia, with every mmol/L increase in total cholesterol associated with a relative risk of 1.11 of the combined end-point [13]. There are little other data on associations between lipid profiles and other markers of vascular disease such as peripheral arterial disease.
Additional well-established cardiovascular risk factors are more commonly found in HIV-infected individuals such as high smoking rates [5], diabetes and insulin resistance [14] and hypertension [15]. Also, although dyslipidemia is a common problem encountered in delivering care to HIV-infected individuals, it is not the only described adverse effect of antiretroviral medications on the cardiovascular system. HIV-infected individuals have increased carotid artery intermediate thickness compared to HIV-negative controls, and those on HAART have further disturbances in vascular distensibility and compliance [16]. Endothelial function is also abnormal in HIV-infected individuals compared to negative controls, with higher serum levels of markers of endothelial dysfunction such as soluble P-selectin, von Willebrand factor and others [17]. Changes in platelet reactivity among HIV-infected individuals have been reported1, and increased insulin resistance and higher prevalence of diabetes is a well described side effect of exposure to some ARV [7, 18]. All of these factors likely act in combination with dyslipidemia to increase overall cardiovascular risk for those infected with HIV.
THERAPY FOR HIV INFECTION
Conventional HAART consists of a combination of three medications drawn from three main drug classes; nucleoside reverse transcriptase inhibitors (NRTIs - nucleoside [or nucleotide] analogues which inhibit the viral reverse transcriptase (RT) enzyme) [19], non-nucleoside reverse transcriptase inhibitors (NNRTIs - which also inhibit the RT enzyme) and protease inhibitors (PIs - which act on the HIV protease) [20]. Traditionally HAART combines two NRTIs with either a NNRTI, or a PI [21]. The decision on which dual NRTI combination or ‘backbone’ to use, and which agent to combine it with, is dependent on numerous factors, including CD4+ T-cell count, HIV viral load, potential toxicities, drug interactions, pill burden and viral resistance.
PATTERNS OF DYSLIPIDEMIA IN HIV
HIV Infection
Prior to the introduction of HAART, it was recognised that HIV infection itself caused dyslipidemia [6]. Declines in total cholesterol, low density lipoprotein cholesterol (LDL-C) and high density lipoprotein cholesterol (HDL-C) have been shown in men who seroconverted from HIV-negative to HIV-positive [22]. HIV-infected, untreated patients (particularly those with more advanced disease) are more likely to have low total, LDL-C and HDL-C and elevated serum triglyceride (TGs) than HIV-negative controls [23, 24, 25], with lower HDL-C concentrations associated with higher circulating HIV RNA levels and longer duration of HIV infection [26, 27].
Antiretroviral Therapies
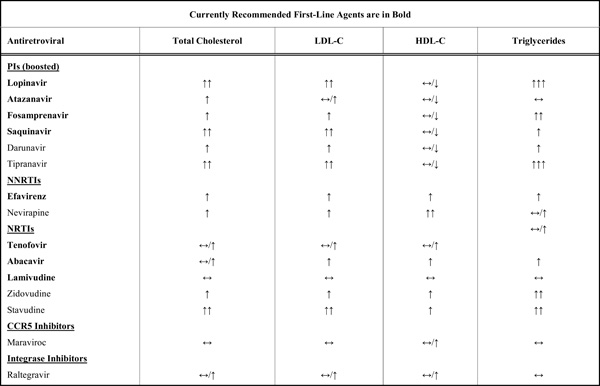
A summary of the overall effects of antiretrovirals on serum lipids are listed in Table 1. Effective HAART suppresses HIV RNA to undetectable levels, allowing immune recovery, measured by increases in CD4+ T-cell counts, in the majority of patients. This is usually accompanied by some increases in total cholesterol and LDL-C [22, 28] which some have suggested may be a return to norm. However, with some PI based therapies, HDL-C levels remain low [22, 29], and hypertriglyceridemia may in fact worsen [30], giving rise to a distinctly atherogenic lipid profile [31]. In contrast, initiation of NNRTI based HAART regimens has been shown to result in increases in HDL-C of approximately 40% depending on the agent used, with increases in total cholesterol, LDL-C and triglycerides also seen, although the triglyceride increases are usually not as severe as those seen with some PIs [32].
Overall Effects of Main Antiretrovirals on Lipid Profiles
 |
LDL-C Treatment Goal Recommendations
| Recommending Group | Risk <10% | Risk 10-20% | Risk <20% Previous CV Disease Diabetes |
|---|---|---|---|
| EACS | <5.0mmol/L (<190mg/dL) | <4.0mmol/L (<155mg/dL) | <3.0mmol/L (<115mg/dL) |
| IDSA/ACTG | <4.1mmol/L (<160mg/dL) | <3.3mmol/L (<130mg/dL) | <2.6mmol/L (<100mg/dL) |
Antiretroviral Switch Studies and Effect on Lipids
 |
Trials of Lipid Lowering Agents in HIV Infection
 |
Many ARVs have dyslipidemic properties. Use of PIs has been associated with hypertriglyceridemia and hypercholesterolemia. Ritonavir, a PI, is a potent inhibitor of the hepatic cytochrome P4503A4 enzyme [33] used at a low dose with other PIs for its ability to ‘boost’ the drug concentrations of the PI, resulting in increased total drug exposure and half-life, allowing for improved dosing regimens and reduced pill burden [34]. In healthy volunteer studies, even when used at a boosting dose (100mg twice daily), exposure to ritonavir increased triglycerides by 26% and LDL-C by 16% after only 2 weeks of therapy [35]. When combined with other protease inhibitors such as lopinavir, serum triglycerides increased by 83%, free fatty acids by 30% and VLDL-C by 33% in HIV-negative subjects after 4 weeks exposure [36]. Similar effects have also been seen in cohorts of HIV-infected patients treated with PIs, including newer drugs such as tipranavir [29, 37, 38]. Induction of dyslipidaemia does not occur with all PIs however. Atazanavir is an azapeptide PI with relatively few effects on serum lipids [39], while other newer PIs such as darunavir have also been shown to induce less dyslipidaemia [40].
Although NNRTIs induce less dyslipidemia than PIs it does appear that efavirenz, one of two commonly prescribed NNRTIs, has a deleterious effect on lipids when compared to the other commonly used NNRTI, nevirapine. The 2NN study compared HAART comprising efavirenz, nevirapine or both in combination with 2 NRTI (stavudine and lamivudine) and demonstrated greater increases in triglycerides in the efavirenz arm compared to the nevirapine arm (49% versus 20% at 48 weeks) [41]. In addition, the AIDS Clinical Trials Group (ACTG) 5142 trial, comparing therapy with lopinavir (PI) or efavirenz (NNRTI) or both with 2 NRTI, demonstrated significant increases in serum triglycerides and total cholesterol in both arms. The combination of lopinavir and efavirenz led to greater increases in serum triglycerides, non-HDL-C, and HDL-C than with either agent alone. Between lopinavir and efavirenz there was no significant difference in cholesterol levels, and only triglycerides were significantly higher in the lopinavir arm [32]. In the Swiss HIV Cohort, triglycerides tended to decrease in those treated with nevirapine but increase with efavirenz [42]. Overall, these data point to significant potential for efavirenz-induced dyslipidaemia, with increases in total cholesterol similar to some PI, but triglyceride increases that are less than observed with use of some PI.
Use of NRTI is not free of dyslipidaemia. Use of the NRTI stavudine has been associated with a worse lipid profile than the nucleotide reverse transcriptase inhibitor (NtRTI) tenofovir, with significantly larger increases in total cholesterol, LDL-C and triglycerides [43]. Similar effects were seen when tenofovir was compared to zidovudine, with significantly smaller increases in total cholesterol and LDL-C observed with tenofovir use [44]. It is for reasons of toxicities such as these that tenofovir and abacavir are now the preferred first line NRTIs compared to zidovudine or stavudine [21]. Recent data from the ACTG 5202 study in which abacavir-containing HAART was compared to tenofovir-containing HAART suggested that abacavir use was associated with significantly greater increases in median triglycerides (25mg/dl vs 3 mg/dl) and total cholesterol (34mg/dl vs 26mg/dl) than tenofovir at 48 weeks2. Similar results were observed in the HEAT study, which also compared abacavir and tenofovir-containing HAART in treatment-naïve patients; those on abacavir had greater increases in serum triglycerides (64mg/dl vs 38 mg/dl) and total cholesterol (32 mg/dl vs 23mg/dl) at 48 weeks3, though the difference between arms was less at 96 weeks [45].
Although these data point to dyslipidaemia induced by ARV from all of the three commonly used drug classes, not all patients respond similarly to antiretroviral regimens (ARV). Cohorts of treatment experienced patients tend to have had more exposure to various classes of ARVs and have greater adverse effects compared to treatment naïve patients. For example, in the ARTEMIS trial, treatment naïve patients started on darunavir at 96 weeks had relatively small (12-15%) increases in total cholesterol, LDL-C and triglycerides [40]. Contrast this to the POWER studies, which used darunavir in heavily pretreated patients, where 15% of patients had TG levels of greater than 8.4mmol/L (744mg/dL) [46].
Metabolic Syndrome and HIV-Associated Lipodystrophy
A possible explanation for the differences in dyslipidaemia upon HAART initiation observed in antiretroviral naïve versus heavily pre-treated HIV-infected patients is the changes in lipid metabolism induced by medium to long term exposure to antiretroviral therapy. While direct dyslipidemia induced by protease inhibitors can develop rapidly, other more chronic metabolic changes affecting lipid metabolism can occur with HAART. Shortly after the introduction of combination antiretroviral therapy, a syndrome of subcutaneous lipoatrophy, central adiposity, dyslipidemia, and insulin resistance, termed HIV-associated lipodystrophy (HIVLD) was noted [7]. This was initially associated with PI exposure [7], but subsequently exposure to NRTIs [47], particularly thymidine analogue NRTIs (tNRTIs) such as stavudine [48] and zidovudine [49] were also recognised as being central to the development of this syndrome. Compared to HIV-infected controls without lipodystrophy, individuals with HIVLD tend to have higher total cholesterol, total cholesterol:HDL ratios, LDL-C and triglyceride levels [50, 51].
In addition to HIVLD, in the general population there is concern about the increasing prevalence of the metabolic syndrome among HIV infected individuals. The pattern of abdominal obesity, low HDL-C, high triglycerides, and insulin resistance, all seen in HIVLD, are also components of the metabolic syndrome [52] . The prevalence of the metabolic syndrome in HIV-infected populations has been reported at 4.4% at enrolment in the D:A:D cohort [53], 17-25% in other studies, and as with the general population, prevalence increases with age and BMI [54, 55, 56]. However, the most common features present in HIV-infected individuals are hypertriglyceridemia and low HDL-C [53, 54] and studies that have matched patients for age and BMI have estimated the prevalence of the metabolic syndrome to be similar among HIV-infected and HIV negative individuals [54, 55].
PATHOPHYSIOLOGY OF DYSLIPIDEMIA IN HIV
HIV Infection
Hypertriglyceridemia in untreated HIV-infected patients may be a response to a systemic inflammatory response against persistent viral infection. TG concentrations, and TG clearance time in untreated HIV-infected patients have been shown to correlate with serum interferon-alpha (IFN-α) [23], which is overproduced in HIV infection [57]. In these untreated patients the activity of lipoprotein lipase (LPL) and hepatic lipase, which are both involved in TG clearance from the circulation, are decreased compared to controls. Treatment of hepatocytes in vitro with IFN-α (and other cytokines such as Il-1) causes increases in lipogenesis [58], and hepatic lipogenesis in vivo is higher in HIV-infected individuals [59]. In hepatitis C virus (HCV) infected individuals receiving IFN-α therapy hypertriglyceridemia combined with low HDL-C levels have also been reported [60, 61], with levels returning to normal upon cessation of IFN-α therapy.
The activity of cholesterol ester transfer protein (CETP), which transfers cholesterol esters from HDL-C to apolipoprotein-B containing proteins [62], is elevated in HIV infection, and its activity correlates inversely with serum HDL concentrations [63]. This may help explain why HDL-C levels are lower in HIV infection. Although the reason for elevated CETP activity is still to be determined, CETP functions more efficiently in the setting of high TG levels [64], and this could help explain the increased activity in HIV-infected patients.
Antiretroviral Therapies
Direct Effect
PIs affect different tissues to cause dyslipidemia. In the liver of mice exposed to PI, excess fatty acid synthesis and hepatic steatosis occur after ritonavir exposure, which is associated with intra-nuclear accumulation of sterol response element binding proteins (SREBP), a nuclear transcription factor important for regulation of expression of many lipid metabolism genes [65, 66]. This effect has been replicated in hepatocytes in vitro, with an increase in SREBP-1 nuclear localistion [67]. Also in the liver, PIs appear to inhibit the proteasomal degradation of pre-secretory apolipoprotein B (the protein component of LDL particles) in cultured hepatocytes [68]. Inhibition of proteosomal degradation of SREBP could also explain its accumulation in hepatic nuclei.
An interesting finding which points towards a hepatic cause of dyslipidemia is the fact that hepatitis C virus (HCV) co-infection appears to protect against the development of HAART-associated dyslipidemia [69-71]. This may be due to alterations in hepatocyte lipid secretion – mono-infection with HCV genotype 3a is associated with a lower serum cholesterol but a marked increase in hepatic steatosis [72].
The effect of PIs in adipose tissue is different to their effect in liver. In adipocytes, PIs inhibit lipolysis by impairing LPL activity, which impairs TG uptake into adipocytes, which may contribute to elevated plasma TG levels [73]. PIs also inhibit SREBP-1 nuclear localisation in adipocytes [74], which leads to decreased adipocyte differentiation and which may also inhibit the ability of adipose tissue to store lipids removed from the circulation. In adipocytes PIs have also been shown to reduce expression of peroxisome proliferator-activated receptor gamma (PPARγ) [75], an nuclear receptor important for adipocyte differentiation. As PPARγ is a transcriptional target of SREBP-1, impaired nuclear localisation of SREBP-1 in adipose tissue may contribute to reduced PPARγ activity.
Indirect Effect
At a cellular level, individuals with HIVLD have atrophic subcutaneous fat with smaller adipocytes, macrophage infiltration and evidence of adipocyte apoptosis [76]. It is known that tNRTIs can accumulate within adipocytes [77]. Adipocytes from patients exposed to tNRTIs demonstrate mitochondrial dysfunction, manifested by depletion of mitochondrial DNA (mtDNA) [76, 78] and reduced mitochondrial RNA (mtRNA) expression [79]. This is felt to be due in part to the ability of tNRTIs to inhibit DNA polymerase-γ, the enzyme responsible for replication of mtDNA [80]. Compared to protease inhibitors, which induce dyslipidemia within a short period of time, the effect of NRTIs on serum lipids takes longer. 6 weeks exposure to tNRTIs did not significantly alter lipid parameters in healthy volunteers, though a reduction in mtRNA expression were seen as early as 2 weeks, without reductions in mtDNA [81]. As with PIs, a downregulation of PPARγ expression in adipose tissue was also observed. The effect of this downregulation is potent as it cannot be overcome with the PPARγ agonist rosiglitazone in the presence of ongoing tNRTI use [79]. These molecular effects may explain how NRTIs can inhibit preadipocyte differentiation [82], reduce triglyceride accumulation [83] and increase adipocyte apoptosis [84] in vitro.
As well as their effects on lipid metabolism outlined above, PIs also affect adipose tissue. PIs can accumulate in adipocytes [77], and ritonavir, lopinavir and saquinavir inhibit adipocyte differentiation [85], an effect not seen with atazanavir, a PI associated with less dyslipidaemia [86]. Nelfinavir has also been shown to induce adipocyte apoptosis [87].
The ultimate result of abnormally functioning subcutaneous adipose tissue is reduced storage capacity for circulating lipids resulting in increased circulating free fatty acids [88], reduced adiponectin secretion [89], and lipid accumulation in non-adipose tissues such as liver (hepatic steatosis [90] and hepatic triglyceride accumulation [91]). This, combined with the effects of PIs on lipid metabolism as mentioned in the previous section, likely underlie the hypertriglyceridemia and hypercholesterolemia observed in HIVLD. Clinical studies have shown that a combination of a PI with NRTIs are a greater risk than PIs alone for the development of lipodystrophy and dyslipidemia [92].
MANAGEMENT STRATEGIES
Although dyslipidaemia in HIV-infected patients is common, not all patients with dyslipidemia require lipid lowering therapy. The goal of therapy, as in the HIV negative population, is to reduce an individual’s cardiovascular risk. Therefore treatment of HIV-associated dyslipidemia should be an integral part of a general attempt to improve cardiovascular health, with advice on diet and exercise, smoking cessation, management of hypertension and diabetes where present, and the use of anti-platelet agents where warranted.
Despite this, in a recent retrospective review, HIV-infected veterans in the USA who met NCEP/ATPIII guidelines for dyslipidemia were half as likely to be receiving lipid lowering therapies as HIV-negative controls [93]. HIV-infected patients should be screening for dyslipidemia at HIV diagnosis, annually, and upon starting HAART. An individual’s cardiovascular risk should be assessed by standardised scoring systems such as the Framingham or Joint British Societies score, and treatment instituted for those with previous cardiovascular disease or a high (>20% in 10 year) risk of cardiovascular disease (Fig. 1).
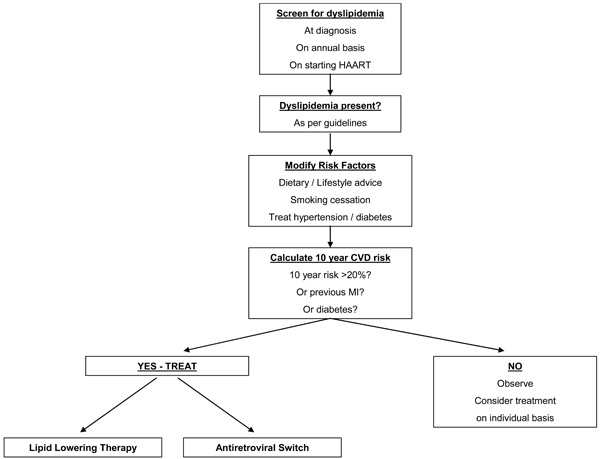
Screening and treatment algorithm.
Current European Aids Clinical Society (EACS) guidelines recommend target LDL-C levels dependent on an estimated 10 year cardiovascular risk. The Infectious Diseases Society of America guidelines use a similar estimate of risk. These are summarised in Table 2.
EACS guidelines recommend only treating severe isolated hypertriglyceridemia (TGs >10mmol/L), or combined hyperlipidemia (LDL-C elevated and TG 2.3-10mmol/L) with lipid lowering therapy, while treating isolated hypertriglyceridemia with TG<10mmol/L with dietary and lifestyle advice. IDSA recommend treatment of isolated TGs>5.6mmol/L (500mg/dL) or elevated LDL-C and TGs of 2.2-5.6mmol/L (200-500mg/dL).
The treatment options available for the management of dyslipidemia in HIV-infection are similar to those in the general population; dietary and lifestyle modification and the use of lipid lowering medications. An additional option in those on HAART is the ability to switch antiretrovirals away from those that may exacerbate the dyslipidemia. Although all these options have been studied in HIV-associated dyslipidemia, many studies are open-label, few are randomised and even fewer placebo-controlled. While many of these studies have shown beneficial effects of switching HAART components or lipid lowering therapy on dyslipidemia, there are no data available on the impact of these effects on cardiovascular disease endpoint and any perceived benefit is derived from corresponding beneficial effects on CVD of lipid lowering in the general population.
Dietary/Lifestyle Modification
As in the management of dyslipidemia in HIV-negative subjects, counselling on ‘therapeutic lifestyle changes’ such as reducing fat and cholesterol intake, increasing physical activity, weight reduction and adjusting diet to reduce LDL-C are also important components of managing HIV-associated dyslipidemia [94], although the data on a beneficial result are inconsistent. Despite similar caloric intake, HIV-infected individuals have been shown to ingest more saturated fat than HIV-negative matched controls, and dietary saturated fat intake correlates with serum triglyceride concentrations [95]. In an early study in PI-treated patients with dyslipidemia, a diet and exercise program led to an 11% reduction in total-C, and a 21% reduction in TG [96]. However, in a study examining the effect of fish oil on serum lipids in HAART treated subjects with hypertriglyceridemia, those randomised to dietary and exercise counselling alone only reduced TG levels by 6%, with no change in LDL cholesterol at 16 weeks despite decreased caloric and total fat intake [97]. In another study of individuals with hypertriglyceridemia on HAART, only 4 of 49 individuals achieved normal TG levels at any stage within an 18 month period with diet and exercise alone [98]. It is therefore unlikely that lifestyle modifications alone will suffice in correcting HAART-induced dyslipidemia in the presence of ongoing exposure to the antiretrovirals precipitating the problem.
Switch Strategies
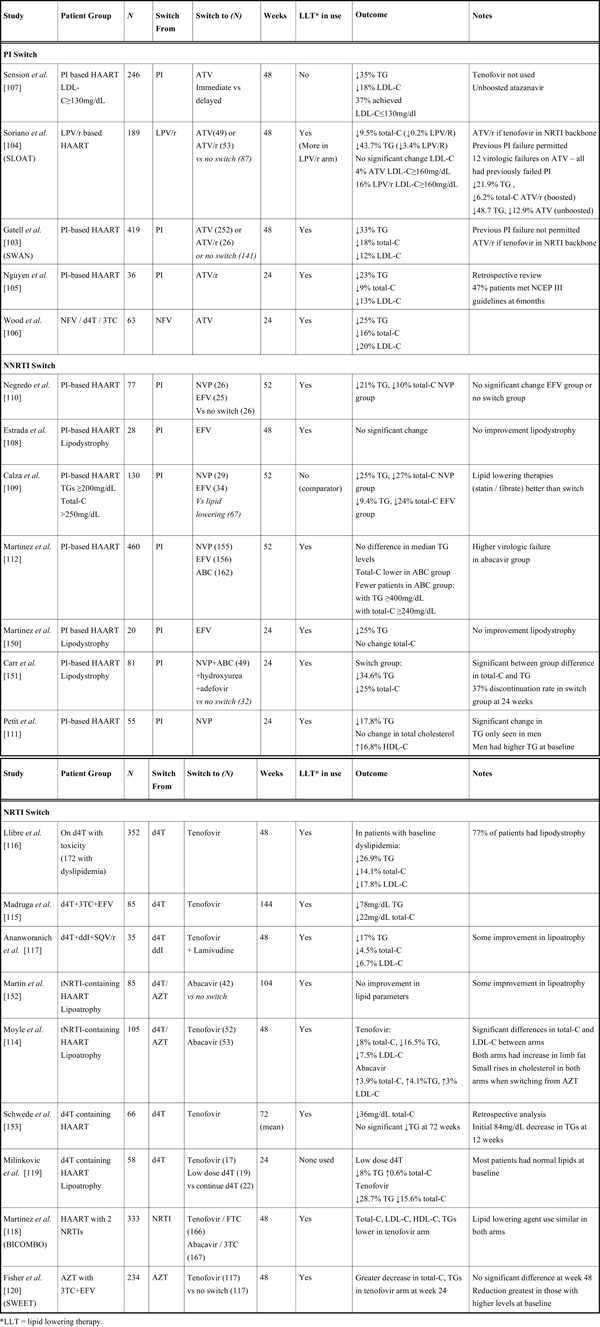
An approach available to some HAART-treated patients with dyslipidemia is to switch away from the presumed offending agent to another ARV with less propensity to induce dyslipidemia [99]. Studies examining this strategy are summarised in Table 3. These options should only be considered when there is a viable alternate antiretroviral agent or regimen, the new regimen is likely to induce less dyslipidemia than the original and is likely to possess similar or enhanced antiretroviral potency compared to the original regimen. At all times, maintenance of an effective ARV regimen capable of durable viral suppression is of paramount importance. Switching therapy needs careful discussion with patients about potential risks and benefits of changing what may be a virologically successful ARV regimen.
PI Switches
Within Class Switching
Switching from one PI to another has the advantage of maintaining the same class of drug whilst preserving future treatment options. Atazanavir is a potent PI with once-daily dosing and relatively favourable effects on serum lipids [39], making it an attractive option to switch to from other PIs. The need to “boost” atazanavir by using boosting doses of ritonavir [100], may reduce the beneficial effects on lipids. However, even when ritonavir-boosted, atazanavir induces less dyslipidemia than other boosted PIs [101], with the possible exception of the newer agent darunavir, which appears to have similar effects on lipid parameters to atazanavir in HIV-negative individuals [102].
Many studies examining switching to atazanavir did not actively recruit patients with dyslipidemia [103, 104, 105, 106]. However there appears to be a consistent reduction in triglycerides and cholesterol among those who switched compared to those who remained on other PIs (Table 3). This appears to hold true even for those who switch to boosted atazanavir, though the margin of reduction in lipids is smaller [104]. Atazanavir can also be used ‘un-boosted’. One study that examined the effect of switching to un-boosted atazanavir in patients with hypercholesterolemia demonstrated reductions in triglycerides and total cholesterol without concomitant lipid-lowering therapy [107]. However only 37% of patients achieved LDL-C ≤130mg/dL.
Switching to a Different ARV Class
Until recently, the options for switching away from protease inhibitors had been confined to NRTI or NNRTI switches. Switching a PI to a NNRTI such as efavirenz or nevirapine makes virological sense, as the combination of 2 NRTIs and one NNRTI is a well studied and effective antiviral combination [99]. However NNRTI have a low genetic barrier to resistance, with only one mutation required to induce high level resistance, raising concerns regarding the efficacy of this approach, particularly in patients who have been previously exposed to NNRTIs. It appears that switching to nevirapine compared to efavirenz has a better effect on lipids [108, 109]. Studies have shown that switching a PI to nevirapine has a minor effect on lipids and is virologically safe [109, 110, 111], though the magnitude of the lipid-lowering effect has been shown to be inferior to lipid lowering therapy alone [109].
Switching a PI to a NRTI such as abacavir, leaving patients on a triple combination of NRTIs, while well-tolerated and beneficial from a lipid perspective, has been shown to be virologically inferior to conventional HAART comprising drugs from more than one drug class [112, 113] and is now not generally recommended.
NRTI Switches
As previously discussed, thymidine analogue NRTIs (tNRTIs) are associated with subcutaneous fat loss, adipose tissue dysfunction, and increases in cholesterol and triglycerides (HIV-associated lipodystrophy). Switching away from tNRTIs to other NRTIs such as abacavir and tenofovir has been investigated as a therapeutic option for HIVLD and have resulted in both increases in subcutaneous fat [114] and improvements in dyslipidemia [115].
Switching from tNRTI to tenofovir appears to have more favourable effects on lipids than switching from tNRTI to abacavir, though the overall lipid improvements are often slight [115, 116, 117]. In one comparative randomised trial, switching from stavudine to tenofovir rather than abacavir resulted in significantly greater improvements in total cholesterol (median reduction of 0.45mmol/L) and LDL-C (median reduction of 0.25 mmol/L) [114]. Similarly in the BI-COMBO study, in which patients were randomised to switch from any NRTI to either tenofovir or abacavir, those switching to tenofovir had lower fasting total cholesterol, LDL-C, HDL-C and triglycerides at week 48 than those switching to abacavir [118]. However the between group differences were relatively small and there was no difference in the use of lipid lowering agents at the end of the study. One study has examined dose reduction of stavudine compared to switching to tenofovir. Despite most patients having normal lipids at baseline, switching to tenofovir resulted in greater lipid improvements than stavudine dose reduction [119]. The SWEET study examined the effect of switching from zidovudine to tenofovir. Although those switching to tenofovir had lower total cholesterol and triglycerides at 24 weeks (with the greatest reductions occurring in those with the highest baseline values), there were no statistically significant between group differences at 48 weeks between each arm [120].
Treatment Interruption
Cessation of HAART has been proposed as a therapeutic option in dyslipidemia, and does result in improvements in TG, total-C and LDL-C even after a short period of time [121]. However, clinical studies have shown excess morbidity and mortality, including increased major cardiovascular, renal and hepatic events in those undergoing treatment interruption [122]. As a result this strategy should not be considered as therapy for dyslipidemia.
Lipid Lowering Therapies
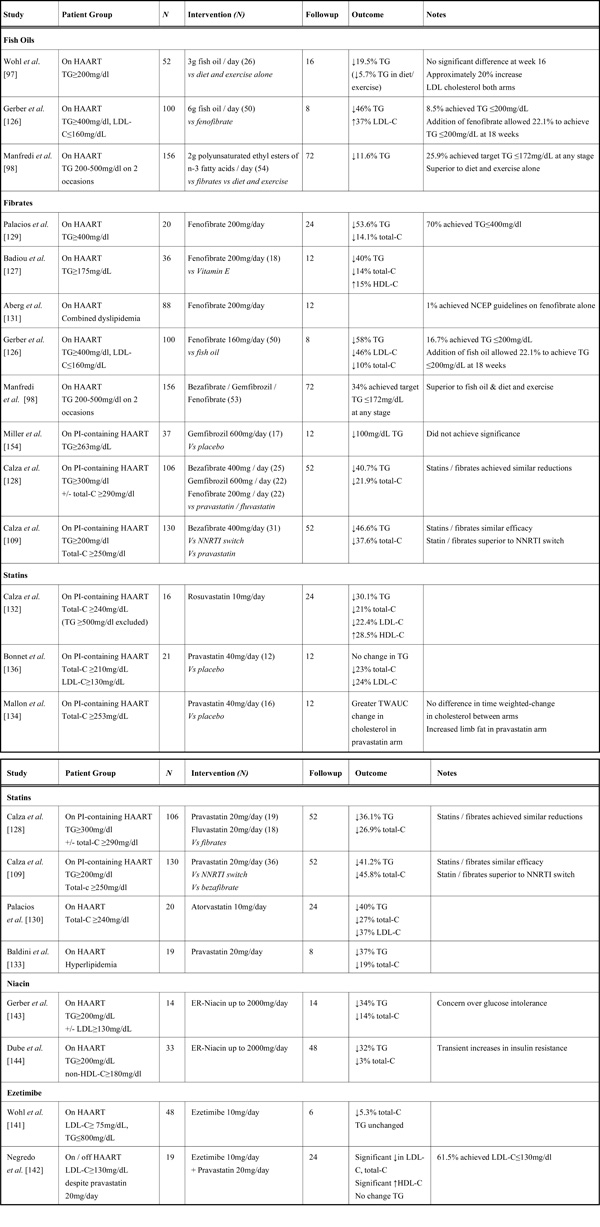
Many lipid lowering therapies have been investigated in HIV infection (summarised in Table 4). The treatment of dyslipidemia in HIV infection is often complicated by potential drug interactions between ARV and lipid-lowering medications. In addition, other medications used in the treatment of HIV-infection such as macrolide antibiotics [123], azole antifungals [124] or the rifamycin antimycobacterials [125] may also have significant interactions with lipid-lowering agents.
Fish Oils
Fish oils, rich in omega-3 fatty acids, can help lower triglycerides and have been studied in HIV-infected patients. They are generally well tolerated and have few side-effects. Although a randomised study of 3g of fish oil daily together with diet and exercise resulted in a 19.5% mean decrease in TGs at 16 weeks (though this was not statistically significant compared to diet and exercise alone), LDL-C concentrations actually increased by 22% [97]. In a further study (ACTG 5186), comparing 6g of fish oil per day to fenofibrate in HIV–infected patients on HAART with isolated severe hypertriglyceridemia, those on fish oil experienced a 46% reduction in median TG at 8 weeks, but only 8.5% achieved TG levels ≤200mg/dL and LDL-C concentrations increased. Combining fish oil with fenofibrate achieved normal TG levels in 22% of patients [126]. Treatment of HIV-infected patients with moderate HAART-related hypertriglyceridemia with polyunsaturated ethyl esters of n-3 fatty acids resulted in a 11.6% reduction in TG levels at 18 months, with 25.9% of patients achieving normal TG levels at some stage over the 18 months follow-up [98]. Given the rises in LDL-C observed in these studies, it is unclear if use of fish oils in this clinical setting will have an overall clinical benefit in terms of cardiovascular risk reduction.
Fibrates
Fibrates are an attractive therapeutic option in HIV-infected patients, as hypertriglyceridemia is common and fibrates lack significant pharmacologic interactions with ARV. Fibrates reduce triglycerides by approximately 40-50% in HIV-infected patients with hypertriglyceridemia [109, 127, 128, 129], with larger reductions in those with higher baseline TG levels [126, 130]. These effects appear to be similar to the effect of statins [128] (see below) and in one study were superior to switching PI to NNRTI [109]. In studies fibrates appear to be well tolerated, with gastrointestinal upset being the most frequently reported side effect. Proportions of patients achieving target goals vary, with one study reporting 70% achieving a target TG≤200mg/dL, but most others report much lower proportions ranging from 1-40% [98, 126, 131]. Despite these benefits, it is still unclear if reductions in triglyceride concentrations alone will be sufficient to significantly alter overall cardiovascular risk in HIV-infected patients.
Statins
HMG CoA reductase inhibitors, or “statins” have been shown to be an effective method of treating hypercholesterolemia in HIV-negative individuals and are also effective in HIV-infected individuals, reducing total cholesterol by approximately 30% and triglycerides by similar amounts [128, 130, 132, 133]. As with use of fibrates for hypertriglyceridaemia, the percentage of patients achieving LDL-C targets with statin use vary, with some studies reporting 50-60% patients reaching NCEP targets [128, 130, 132], while others report far lower percentages [131, 134]. Interactions between statins and antiretrovirals (ARVs) are very common. PIs inhibit the CYP3A4 metabolism of statins, and increase overall exposure to statins, in the case of simvastatin by over 3000% [135]. This has led to concerns regarding the potential for statin toxicity with PI use and for this reason simvastatin is contraindicated with concurrent PI use. However atorvastatin (at 10-80mg qd) and pravastatin (at 20-80mg qd) are less affected by this interaction and have been extensively studied with PIs [109, 128, 130, 136]. Conversely, the NNRTI efavirenz induces hepatic metabolism of statins, leading to a marked reduction in the AUC for simvastatin, pravastatin and atorvastatin [137]. The newer statin rosuvastatin is only marginally metabolised by the CYP3A4 system, with most of the drug being excreted unchanged, and has been used to treat dyslipidaemia in HIV-infected patients [132]. Despite this, both lopinavir/ritonavir [138, 139] and atazanavir/ritonavir [140] increase both the Cmax and AUC of rosuvastatin. This may be due to blockage of the OATP-1B1 membrane transporter for rosuvastatin, of which ritonavir is a known inhibitor [138]. This transporter is involved in the hepatic uptake of rosuvastatin, therefore ritonavir inhibition may reduce the effect of rosuvastatin on lowering cholesterol despite inducing higher circulating concentrations of the drug [124].
Ezetimibe
Ezetimibe, which blocks cholesterol absorption in the intestine and is metabolised independent of the CYP3A4 pathway, appears to have minor effects in HAART-induced dyslipidemia as monotherapy [141]. However when added to pravastatin it allowed 62% of patients with hyperlipidemia to achieve a target LDL-C concentration of ≤130mg/dl [142]. Its effects seem confined to LDL-C reduction as effects on triglycerides in HAART-induced dyslipidemia appear minor [141, 142].
Niacin
Niacin has been investigated in two small studies, and appears to be well-tolerated, reducing TG levels by approximately 30% with a smaller effect on total cholesterol [143, 144]. A concern is the increase in insulin resistance seen in both studies. Given the concern over insulin resistance and diabetes in HIVLD the role for routine use of niacin in this setting is unclear.
Combination Therapies
There are few data reporting the efficacy or safety of combination therapies in HIV-infected individuals. One open label study allowed the addition of pravastatin or fenofibrate to those who had failed to reach NCEP guidelines on either agent as monotherapy for 12 weeks [131]. Combination therapy was well tolerated and led to reductions in TG levels particularly, but only allowed 4% of patients to achieve combined NCEP guidelines. The increased risk of rhabdomyolysis in HIV-negative individuals from the combination of statins and fibrates [124] would advise their concurrent use in HIV positive patients only be undertaken with strict caution.
CONCLUSION/FUTURE DIRECTIONS
As the ongoing roll-out of antiretroviral treatment programs in resource poor settings is continuing, use of older medications such as tNRTIs is common [145]. As a result dyslipidemia and lipodystrophy will continue to be major issues for many HAART treated patients for years to come [146]. The recent introduction of new medications with more lipid friendly profiles within existing classes such as darunavir (PI) [46], and etravirine (NNRTI) [147] will broaden the options available to clinicians. In addition, entirely new classes of drugs, such as integrase inhibitors (raltegravir) [148] and CCR5 inhibitors (maraviroc) [149] should allow more options both for antiretroviral-naïve patients starting therapy and those needing to switch therapies to avoid dyslipidaemia. Furthermore, the development of newer, selective boosting agents to replace ritonavir that lack dyslipidaemia is continuing4. Such advances could reduce the requirement for ritonavir as a boosting agent, further reducing the potential for ARV to induce dyslipidemia. Despite early data showing much less effects on lipids with these new agents, long term safety data is required and clinicians will need to remain vigilant for treatment-related toxicities as HIV-infected individuals continue to live longer and healthier lives.
NOTES
1 Satchell C, Cotter A, O'Connor E, et al. A Case Control Assessment of Platelet Function in HIV-1+ and HIV-1- Individuals. Abstract 737, Sixteenth Conference on Retroviruses and Opportunistic Infections, Montreal, 2009.
2 Sax P, Tierney C, Collier AC, et al. ACTG 5202: shorter time to virologic failure (VF) with abacavir/lamivudine (ABC/3TC) than tenofovir/emtricitabine (TDF/FTC) as part of combination therapy in treatment-naïve subjects with screening HIV RNA >100,000 c/mL. Abstract THAB0303, XVII International AIDS Conference, Mexico City, 2008.
3 Smith K, Fine D, Patel P, et al. Efficacy and Safety of Abacavir/Lamivudine Compared to Tenofovir/Emtricitabine in Combination with Once-daily Lopinavir/Ritonavir through 48 Weeks in the HEAT Study. Abstract 774, Fifteenth Conference on Retroviruses and Opportunistic Infections, Boston, 2008.
4 Mathias A, Lee M, Callebaut C, et al. GS-9350 : A Pharmaco-enhancer without Anti-HIV activity. Abstract 40, Sixteenth Conference on Retroviruses and Opportunistic Infections, Montreal, 2009.
FUNDING
Eoin Feeney is supported through the Clinician Scientist Fellowship Programme by Molecular Medicine Ireland. The HIV Molecular Research Group is funded through a grant by Science Foundation Ireland (09/RFP/BMT2461).


