All published articles of this journal are available on ScienceDirect.
High Sensitivity C - Reactive Protein is Associated with Diastolic Dysfunction in Young African Americans without Clinically Evident Cardiac Disease
Abstract
Background:
Diastolic dysfunction (DD) is associated with myocardial fibrosis mediated by inflammation. Higher levels of inflammation found in African Americans (AAs) may predict DD among asymptomatic individuals. We tested the hypothesis that high sensitivity C-reactive protein (hs-CRP), a biomarker of inflammation, is associated with DD in asymptomatic AAs.
Methods:
We prospectively recruited 107 asymptomatic AAs without any history of cardiac, renal or inflammatory diseases or alcoholism. We measured hs-CRP and B-type Natriuretic peptide (BNP) levels and estimated left ventricular end diastolic pressure (LVEDP), mass and systolic function with echocardiography. Multivariate logistic regression analysis was used to define whether hs-CRP is an independent predictor of LVEDP.
Results:
Among 107 subjects: the mean age was 48±10 yrs, 58 (54%) were men, 59 (55%) had diabetes (DM), 48 (45%) had hypertension (HTN), the mean BMI was 30.5±4.8 and the mean ejection fraction was 63.1±5.8%. DD was present in 56(52%) subjects, 38 (36%) of whom also had a high LVEDP. On multivariate analysis, hs-CRP was independently associated with DD [odds ratio 3.36 (95% CI= 1.07 - 10.5, p = 0.04]. There was a 61% and 133% increase in the prevalence of any DD and DD with high LVEDP, respectively, between the lowest and the highest hs-CRP quartiles.
Conclusion:
Diastolic dysfunction is prevalent among asymptomatic African Americans and it is independently associated with elevated level of hs-CRP, an inflammation marker.
INTRODUCTION
Diastolic dysfunction (DD) is the predominant pathophysiological mechanism in a third of the heart failure population [1-6]. Inflammatory fibrosis of the myocardium is thought to be a major cause of myocardial stiffening leading to the development of DD. The higher prevalence of proinflammatory conditions such as diabetes (DM) and hypertension (HTN) and the higher levels of inflammatory markers often found in African Americans (AA) may predispose them to myocardial stiffening and DD, ultimately resulting in symptomatic heart failure [7-11]. We conducted this cross sectional study to test the hypothesis that high sensitivity C-reactive protein (hs-CRP), a biomarker of inflammation, is associated with DD and is a clinical predictor of incipient diastolic heart failure in asymptomatic AA subjects.
METHODS
Study Subjects
We prospectively screened 135 AAs between the ages of 20 and 65 years with no known history of heart disease or cardiovascular symptoms who visited an outpatient walk-in clinic between March 2004 and May 2006 at an urban healthcare center in Chicago. Of those 117 were included in the study (18 declined to participate) (Fig. 1). They presented to the clinic for medications for their underlying chronic conditions or for unrelated medical problems. A standardized interview and physical exam was administered to all subjects. Blood pressure was measured using established methods [12]. HTN was defined as a systolic blood pressure ≥140 mm Hg and/or a diastolic blood pressure > 90 mm Hg or receiving antihypertensive drug therapy [13]. Subjects were classified as having DM if they had a history of type 2 diabetes diagnosed with the American Diabetic Association criteria or were on medications for diabetes [14]. We successfully recruited approximately equal number of subjects who had HTN and DM, DM only, HTN only and neither HTN nor DM as planned.
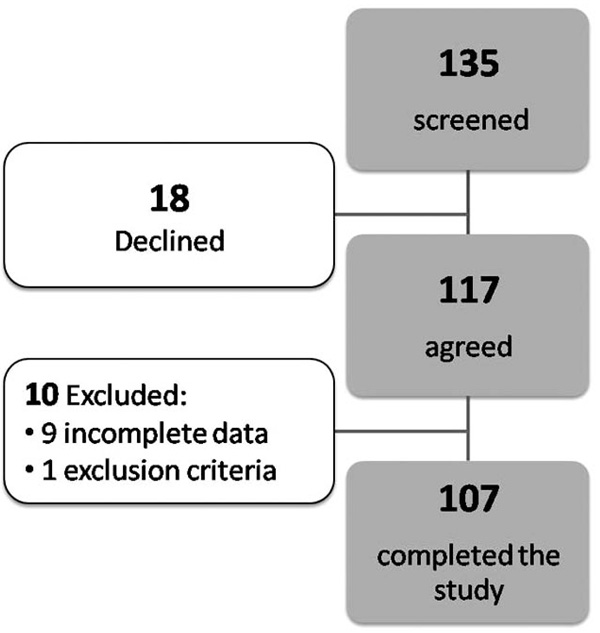
Study Scheme DD - diastolic dysfunction, DM – diabetes mellitus, hs-CRP = high sensitivity C-reactive, HTN – hypertension, LVEDP = left ventricular end diastolic pressure.
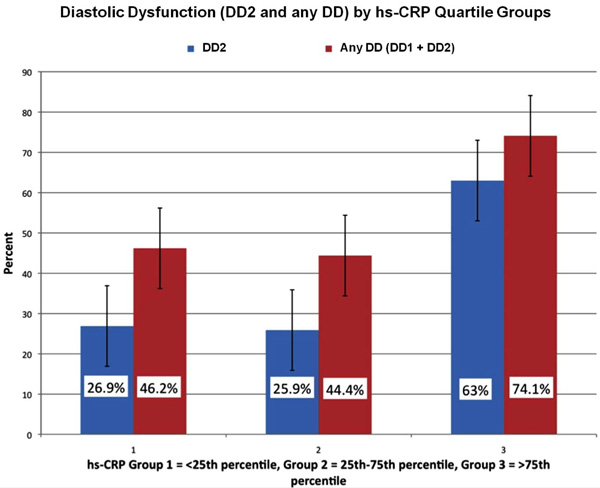
Rates of DD by hs-CRP quartiles. Overall DD and DD2 rates were significantly different among the hs-CRP quartile groups (p=0.03 and p=0.003 respectively). There were significant pair wise differences among the hs-CRP quartile groups 1 and 3 (p=0.008) and groups 2 and 3 (0.001) in the rate of occurrence of any DD.
Subjects were excluded if they had signs and symptoms of heart failure or other cardiac symptoms, history of prior cardiovascular diseases (myocardial infarction, coronary artery disease, heart failure, valvular heart disease, arrhythmia, bundle branch block, wall motion abnormalities on earlier echo, stroke, and peripheral vascular disease), newly diagnosed (less than one year) DM or HTN or type 1 DM, serum creatinine ≥ 1.6 mg/dl, history of cocaine or heroin use in the last 6 months, alcoholism (defined as at least two positive responses to CAGE questionnaire), [15] body mass index (BMI) ≤18.5 or ≥40 and conditions associated with acute hs-CRP elevation (those with signs of acute systemic infection/inflammation such as fever or the use of estrogens in any form) [16-19].
Laboratory measurements included serum creatinine levels, hemoglobin A1C (HbA1c), b-type natriuretic peptide (BNP) (Advia Centaur Immunoassay systems, Bayer diagnostics, New York, USA) and hs-CRP levels (Immulite 2000 immunometric assay, DPC, California, USA). Any value below the lowest possible measurement of BNP (< 0.5 pg/dl) and hs-CRP value (<0.02 mg/dl) was considered zero. All subjects provided written informed consent. The Scientific Research Committee of the hospital approved this study. This human investigation conforms to the principles outlined in the Declaration of Helsinki.
Echocardiography
M-mode, 2D, Doppler and tissue Doppler imaging was performed on all subjects using standard techniques. Ejection fraction and wall motion abnormalities were assessed visually. Measurements of interventricular septal thickness (IVST), posterior wall thickness (PWT) and left ventricular internal diameter (LVID) were made at end-diastole and end-systole according to the American Society of Echocardiography (ASE) recommendations [20]. Left ventricular (LV) mass was calculated using the ASE formula with Devereux’s correction and raised to the power 2.7 (LV mass index-LVMI) to minimize the errors in estimating the impact of overweight [21, 22].
All participants had a pulsed-wave Doppler examination of mitral inflow and of pulmonary venous inflow. Doppler velocities of early (E) and late diastolic mitral flow (A), the deceleration time (DT) and the E/A ratio (including ∆ E/A ratio with Valsalva maneuver) were measured. Pulmonary venous peak systolic flow velocity and diastolic flow velocity were also obtained [23]. Tissue Doppler imaging was obtained at the medial mitral annulus. The early (e′) and late diastolic velocities (a') were determined and the ratio of the transmitral flow velocity and annular velocity (E/e') was performed to assess LV end diastolic pressure (LVEDP). The prediction of elevated filling pressure was primarily based on E/e’ ratio > 10 and a decrease in E/A ratio by 0.5 with Valsalva maneuver [24]. Subjects with E/e' ≤10 were considered to have normal LVEDP [25-27].
Classification of Diastolic Dysfunction
Using standard criteria for Doppler measurements of mitral inflow, pulmonary venous flow and tissue velocities, diastolic function was categorized into normal, DD with normal LVEDP (DD1) (impaired relaxation, also known as grade-1 DD) and DD with high LVEDP (DD2) . The latter group includes subjects with impaired relation with elevated LVEDP (also known as grade-1B), pseudonormal filling pattern (grade-2 DD) and advanced DD (grade 3, restrictive filling pattern) [3, 23]. LVEDP and diastolic function classification were adjudicated by a cardiologist who is an expert in echocardiographic assessment of diastolic function. Rules utilized in predicting LVEDP and diastolic function classification are consistent with the subsequently published recommendations of American Society of Echocardiography [28].
Statistical Analysis
Association of DD1, DD2 or E/e' ratio with hs-CRP was determined controlling for the effects of possible confounders (age, sex, diabetes, hypertension, BMI and LVM index) and that of BNP. Separate logistic regression analyses were used to determine association of DD1 or DD2 with hs-CRP, BNP, age, sex, BMI, LVM index, DM, or HTN unadjusted for any other factor. Multiple logistic regression analysis was used to determine association of DD1 or DD2 with hs-CRP controlling for the effects of significant confounders and BNP. The assumptions and validity of the logistic regression model was ascertained (model fitting information, goodness of fit). Spearman rank correlation was used to determine unadjusted correlation between E/e' ratio and hs-CRP while the Wilcoxon rank sum test was used to determine association of E/e' ratio with sex, DM and HTN by comparing average value of E/e' ratio between categories of these possible confounders. The general linear model was used to determine significant association between E/e' ratio and hs-CRP adjusted for the effects of significant confounders and BNP. The quartiles for hs-CRP were compared for prevalence of (DD1) and (DD2) using the chi-square test.
RESULTS
In all, of 117 subjects recruited, 107 completed the study procedures (9 had incomplete data and 1 met exclusion criteria, Fig. 1). The baseline characteristics are presented in Table 1. Echocardiogram revealed any DD in 56 (52%) of these asymptomatic individuals: 18 (17%) had mild DD (DD1) with normal LVEDP (E/e′ ratio ≤10) and 38 (36%) had DD with elevated LVEDP (DD2) [E/e′ ratio >10 and a decrease in E/A ratio by 0.5 with Valsalva maneuver].
Baseline Characteristics
| Characteristics | Participants (n = 107) |
|---|---|
| Age (yrs) (mean ± SD) | 48±10 |
| Men | 58 (54%) |
| Diabetic not hypertensive | 32 (30%) |
| Hypertensive not diabetic | 21 (20%) |
| Diabetic hypertensive | 27 (25%) |
| Not diabetic not hypertensive | 27 (25%) |
| Body mass index (kg/m2) (mean ± SD) | 30.5±4.8 |
| Systolic blood pressure (mm Hg) (mean ± SD) | 124±17 |
| Diastolic blood pressure (mm Hg) (mean ± SD) | 78±11 |
| Glycosylated hemoglobin HbA1c (%) (mean ± SD) | 7.9±2.5 |
| hs-CRP levels (mg/dl) (mean) (range) | 0.5 (0.02-4.65) |
| hs-CRP levels (mg/dl) (<25th percentile) | ≤0.1 |
| hs-CRP levels (mg/dl) (25th-75th percentile) | 0.11 – 0.61 |
| hs-CRP levels (mg/dl) (>75th percentile) | ≥0.614 |
| BNP levels (pg/dl) | 18.7 (0-180) |
| LV mass (gm) | 158±49 |
| LVMI (gm/m2.7) | 38±11.4 |
| Ejection fraction (mean ± SD) | 63.1±5.8% |
BNP = b-type natriuretic peptide, hs-CRP = high sensitivity C-reactive protein, LV = left ventricle, LVMI = left ventricular mass index.
Factors Associated with Diastolic Dysfunction by Univariate Analysis
| Factors Significantly Associated with Outcomes Odds Ratio (95% CI) | p-value | |
|---|---|---|
| DD1 | Age: OR=1.19 (1.11 to 1.27) | <0.01 |
| HTN: OR=7.14 (2.94 to 16.67) | <0.01 | |
| LVM Index: OR=1.07 (1.03 to 1.12) | <0.01 | |
| Hs-CRP: OR=3.94 (1.32 to 11.72) | 0.014 | |
| DD2 | Age: OR=1.12 (1.06 to 1.19) | <0.01 |
| HTN: OR=5.56 (2.38 to 12.50) | <0.01 | |
| BMI: OR=1.11 (1.02 to 1.21) | 0.02 | |
| LVM Index: OR=1.09 (1.04 to 1.14) | <0.01 | |
| Hs-CRP: OR=4.35 (1.61 to 11.77) | <0.01 | |
| E/e’ | Age: Spearman rank correlation coefficient = 0.423 | <0.01 |
| LVM: Spearman rank correlation coefficient = 0.402 | <0.01 | |
| BMI: Spearman rank correlation coefficient = 0.234 | 0.02 | |
| Hs-CRP: Spearman rank correlation coefficient = 0.340 | <0.01 | |
| HTN: 10.8±3.3 (Yes) vs. 8.3±2.9 (No) [comparison of means] | <0.01 |
BMI = body mass index, Hs-CRP = high sensitivity C-reactive protein, HTN = hypertension, LVMI = left ventricular mass index, DD = diastolic dysfunction.
Factors Associated with Diastolic Dysfunction after Adjusting for the Effect of Confounders
| DD Outcome | Factors Associated with DD Adjusted for the Effects of other Factors | p-value |
|---|---|---|
| DD1 | hs-CRP: OR=3.36 (1.07 to 10.52) | 0.04* |
| Age: OR=1.16 (1.08 to 1.25) | <0.01** | |
| LVM: OR=1.04 (0.99 to 1.10) | 0.15NS | |
| HTN: OR=2.17 (0.72 to 6.25) | 0.17NS | |
| DD2 | hs-CRP: OR=3.62 (1.78 to 10.23) | 0.02* |
| Age: OR=1.10 (1.03 to 1.17) | 0.006** | |
| LVM: OR=1.07 (1.01 to 1.13) | 0.02* | |
| HTN: OR=1.65 (0.55 to 5.00) | 0.36NS | |
| BMI: OR=0.99 (0.89 to 1.11) | 0.84NS | |
| DD3 | hs-CRP: MS1=61.03, F2=7.89 | 0.006** |
| Age: MS=61.35 , F=7.93 | 0.006** | |
| LVM: MS=45.76, F=5.92 | 0.02* | |
| HTN: MS=7.97, F=1.03 | 0.31NS | |
| BMI: MS=.04, F=.01 | 0.94NS |
** Significant at 1% level of significance (p-value<0.01)
* Significant at 5% level of significance (0.01<p-value<0.05)
NS Not Significant at 5% level of significance (p-value>0.05)
1 MS – Mean Square for factor in general linear regression model
2 F – Ratio of MS (factor) to mean square error for testing significant effect of factor on E/e′ ratio BMI = body mass index, Hs-CRP = high sensitivity C-reactive protein, HTN = hypertension, LVMI = left ventricular mass index, DD = diastolic dysfunction
Univariate analyses of various factors associated with DD1 and DD2 were made and the results are shown in Table 2. Age, BMI, HTN and LVMI were significant confounding factors for the association between hs-CRP and DD2 and E/e′ ratio. These same factors except BMI were the significant confounding factors for the association between hs-CRP and DD1. There was no significant association between BNP and DD1 (p=0.66), DD2 (p=0.16) or E/e′ ratio (p=0.61) and hence were not included in the multivariate analysis.
Table 3 shows significant association between hs-CRP and DD1 (p=0.04), DD2 (p=0.02), and E/e′ ratio (p=0.006) adjusted for the effects of confounding factors in the multivariate analysis. For every unit increase in hs-CRP, there were significant increases of 3.36% and 3.62% in odds for DD1 and DD2, respectively, adjusted for the effect of confounding factors. There was a significant contribution of hs-CRP to the observed variability of E/e′ ratio (F ratio of mean square for hs-CRP to mean square for error=7.93) controlling for the effects of confounding factors. Independent risk factors for DD2 and E/e′ ratio were age, LVM index and hs-CRP whereas only age and hs-CRP were the independent risk factors for DD1.
There was an overall significant difference in overall all prevalence of any DD (p=0.03) and DD2 (p=0.003) rates among the hs-CRP quartile groups. There were significant pair wise differences among the hs-CRP groups 1 and 3 (p=0.008) and between groups 2 and 3 (0.001) in rate of occurrence of DD (Fig. 2).
Of all the participants 3 were found to have mild upper respiratory infection with no fever or systemic symptoms. Exclusion of these participants from the analysis did not change the outcome.
DISCUSSION
Myocardial stiffness and DD can result from cardiac fibrosis caused by the increased deposition of collagen in the interstitium mediated in part by inflammatory processes [1, 29, 30]. Small studies have suggested that low-grade systemic inflammation is associated with high arterial stiffness, which in turn is associated with the stiffening of the ventricle and diastolic heart failure [31-34]. African American subjects have a higher prevalence of proinflammatory conditions like DM and HTN, which puts them at a risk of an earlier onset and accelerated progression of arterial stiffness and are at high risk for diastolic dysfunction [35].
In the present study of asymptomatic AA subjects, the prevalence of DD including those with high LVEDP was very high (56%). DD to this degree in a relatively young population may be of considerable clinical importance, since the presence of DD is a powerful predictor of symptomatic heart failure and all-cause mortality [3, 36]. The most important finding of the present study is the strong association between DD and elevated hs-CRP levels, independent of DM, HTN, BMI, and LV hypertrophy. It is also important to note that those with higher hs-CRP levels tend to have more advanced DD with elevated LVEDP. Others have reported an association between hs-CRP levels and LVEDP in patients with heart failure [37]. Our data suggests that this association appears to exist even in those with asymptomatic DD. The strong independent association between hs-CRP levels, a well-established marker of inflammation and DD may indicate that inflammation plays a prominent role in the development of DD. It was also interesting to note that the BNP levels were not significantly associated with DD in this asymptomatic population. It has been noted by others as well that in asymptomatic population with diastolic dysfunction BNP may not always be elevated [38, 39].
We conclude that hs-CRP appeared to be an important clinical predictor of DD in this cohort independent of the other clinical predictors of DD like age, HTN, DM and BNP. Therefore, it may be another potential biomarker of incipient heart failure.
Mechanism of Association between Inflammation and DD
Myocardial fibrosis in diastolic heart failure is characterized by poorly structured and fractured collagen in the extracellular matrix (ECM) [10, 29, 40, 41]. Matrix metalloproteinases (MMP) are enzymes released by macrophages and fibroblasts, which modulate the remodeling of the ECM [41, 42]. These MMPs and the tissue inhibitors of matrix metalloproteinases (TIMPs) are in turn regulated by inflammatory cytokines such as C-reactive protein [41, 43, 44].
Further support to the etiological role of inflammation in causing DD comes from animal studies where normalization of DD was achieved using anti-inflammatory agents independent of the resolution of LV hypertrophy [45, 46]. Inflammation has been proposed to be a common pathway through which conditions like HTN and DM lead to ventricular stiffness and DD and eventually to decompensated heart failure.
Alternatively it can be speculated based on current body of evidence that elevated hs-CRP may be either a marker or mediator of impaired coronary blood flow reserve which is known to be associated with ventricular fibrosis and diastolic dysfunction [47-49].
With the increase in DM, HTN and obesity, the prevalence of DD is likely to increase in the near future. To date, the management of DD in clinical practice is based mostly on our knowledge of the pathophysiology and trial data in established diastolic heart failure [50]. There are few specific interventions to reverse DD and to prevent the development of symptomatic heart failure once DD is detected. If the association between inflammation and DD can be confirmed by further study in humans, controlling inflammation could be a target for therapy for DD and possibly for the prevention of symptomatic heart failure [51]. The hs-CRP level also has the potential to be useful in identifying those at risk for DD and possibly predict those who are likely to progress to symptomatic heart failure, pending confirmation by prospective and long-term observational studies.
STUDY LIMITATIONS
We recruited patients for this study from an outpatient walk-in clinic of an inner-city hospital. Many patients visit this facility to obtain medication refills for their underlying chronic condition. This may explain the high proportion of patients with type-2 DM, as these individuals may have disproportionately frequented the clinic for the sole purpose of obtaining a medication refill. Additionally, this fact would suggest that patients recruited may have been sub-optimally managed for their underlying chronic condition as they utilized an urgent care center rather than a steady primary care physician to treat these conditions. Possibly, we may have included patients who ran out of their antihypertensive or DM medications with subsequent acute changes in their blood pressure or glucose level altering their diastolic function [52]. We acknowledge the possibility of these factors affecting the prevalence of DD among the enrolled subjects. Nevertheless, these selection biases do not explain the observed correlation between DD, elevated LVEDP and hs-CRP in asymptomatic patients. Furthermore, the biological plausibility reasoning we outlined in our discussion justifies further examination of our findings.
This was a cross sectional study with a single measurement of hs-CRP levels. Hence this study did not capture inherent variability of hs-CRP levels introduced by moderate alcohol consumption, increased activity and exercise, medications and other unknown variables or linear trends in hs-CRP levels [53]. This limitation may explain the presence of DD in those with low hs-CRP levels. Alternatively there may be other markers of inflammation (e.g. IL-6) or collagen metabolism that could be more closely associated with DD. This study did not measure any other inflammatory markers or markers of collagen metabolism. It is likely that there are also non-inflammatory factors associated with DD. A prospective longitudinal study with serial measurement of hs-CRP levels and other markers of inflammation and collagen metabolism is needed to address these issues. Also the study population was not tested for underlying coronary artery disease, which could also have an inflammatory origin and present as diastolic dysfunction. It was not considered necessary to subject an asymptomatic population to further risky or invasive testing in the absence of specific therapy other than modifying risk factors. Besides the purpose of this study is to report the association between inflammation and diastolic dysfunction in this population and delineating the mechanism of association is beyond the scope of this study.
CONCLUSION
Our data establishes a strong and independent association between hs-CRP levels and the presence of DD in asymptomatic AAs, which supports the concept of systemic inflammation as an etiological factor in myocardial fibrosis and LV dysfunction. If confirmed by larger, longitudinal studies this association may have implications in identifying the population at risk for heart failure and for possible new targets for treatment of DD and prevention of symptomatic heart failure. Hs-CRP levels may also identify individuals with advanced DD.
ACKNOWLEDGEMENTS
- Philip R. Liebson, MD; Professor of Medicine, Senior Attending Physician, Preventive Cardiology, Rush University, Chicago, IL 60612.
- Ms. Jackie Polk, RN; John Stroger, Jr. Hospital of Cook County, Chicago, IL 60612.
- Ms. Bessie Coleman, RN; John Stroger, Jr. Hospital of Cook County, Chicago, IL 60612.
FUNDING
This study was supported by an internal financial grant from the divisions of Cardiology and Endocrinology at the John Stroger hospital of Cook county, Chicago, IL, USA.
Reagents for measurement of BNP were provided by Bayer diagnostics, New York, USA. Reagents for measurement of hs-CRP were provided by DPC, Los Angeles, California.


