All published articles of this journal are available on ScienceDirect.
Familial Mediterranean Fever as an Emerging Clinical Model of Atherogenesis Associated with Low-Grade Inflammation
Abstract
Numerous inflammatory and innate immune pathways are involved in atherogenesis. Elaboration of clinical models of inflammation-induced atherogenesis may further advance our knowledge of multiple inflammatory pathways implicated in atherogenesis and provide a useful tool for cardiovascular prevention. Familial Mediterranean fever (FMF) is a chronic inflammatory disorder with profiles of inflammatory markers close to that seen in the general population. In a few recent studies, it has been shown that endothelial dysfunction, increased atherosclerotic burden and activation of platelets accompany attack-free periods of FMF. Colchicine is proved to be useful in suppression of inflammation in FMF. Preliminary basic and clinical studies suggest that this relatively safe drug may be useful for cardiovascular protection in patients with FMF and in the general population. Multinational prospective studies are warranted to further elaborate clinical model of inflammation-induced atherosclerosis associated with FMF.
INTRODUCTION
Atherosclerosis is the main contributor to the global morbidity and mortality [1]. It starts early in life, progresses slowly and asymptomatically with aging, eventually resulting in atherosclerotic cardiovascular disease, adverse vascular events and death. Staggering amount of evidence derived from experimental and clinical studies suggests that multiple immune and inflammatory agents orchestrate atherosclerotic vasculopathy throughout the whole course of atherogenesis [2,3].
Initial stages of atherosclerotic vasculopathy are characterized by disruption of the integrity of endothelial monolayer, exposure of the constituents of subendothelial space to the blood flow, recruitment of immune and inflammatory cells into the vascular wall, vascular inflammation and decreased production of vasoprotective agents [4, 5]. An interaction of endothelial cells with T- and B-lymphocytes, neutrophils, platelets and numerous immune and inflammatory agents produced by these cells leads toward atherosclerotic plaque formation, its destabilization and rupture [6-9].
Over the past decade, it has become evident that upregulation of inflammation and autoimmune aggression in certain systemic disorders such as systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA) may substantially accelerate atherogenesis and increase the risk of vascular events [10-12]. Basic and clinical research studies in this field have led to the emergence of a new biomedical discipline, vascular rheumatology [13], which aims to clarify pathophysiology of rheumatic diseases and co-morbidities and to propose recommendations for primary and secondary prevention of vascular events in the general population. Clinical models of inflammation-induced atherosclerosis elaborated over the past decade are mainly based on the cardiovascular phenomenon described within the frames of high-grade inflammatory conditions. Much less attention has been paid to atherogenesis in low-grade inflammatory conditions, such as psoriatic arthritis [14, 15], ankylosing spondylitis [16] and familial Mediterranean fever (FMF) [17, 18] which manifest with inflammatory profiles close to that seen in the general population. Better understanding of diverse inflammatory pathways associated with atherogenesis may provide useful tools for cardiovascular prevention with drugs proved their efficiency in these disorders (for example, colchicine in FMF).
The aim of the current mini-review is to discuss latest data supporting the concept of FMF as a clinical model of atherogenesis associated with low-grade inflammation.
FMF AS A LOW-GRADE INFLAMMATORY DISORDER
FMF has been classified as a systemic autoinflammatory disorder characterized by seemingly unprovoked activation of diverse inflammatory pathways [19, 20]. It is an ancient disease with wide-spread distribution in the Eastern Mediterranean region and sporadic occurrence throughout the world. The etiopathophysiology of FMF is not fully understood.
However, it is largely recognized as an autosomal recessive trait associated with missense mutations in the MEditerranean FeVer (MEFV) gene located on the short arm of chromosome 16 [21]. The gene is predominantly expressed in neutrophils [22]. The mutant pyrin protein encoded by the MEFV gene contains 781 amino acids and 4 domains (pyrin N-terminal, B-BOX type zinc finger, Coiled Coil and B30.2 C-terminal). The B30.2 domain encoded by the last, 10th exon of the MEFV gene which is the location of all severe mutations (M694V, M694I, M680I) [21]. These mutations are responsible for dysregulation of apoptosis and inflammation due to the reduced ability of the mutated pyrin protein to modulate activity of inflammasome and interleukin-1beta (IL-1beta) [19]. Importantly, the same mutations were found responsible for subclinical inflammation in apparently healthy subjects [23, 24] and were associated with myocardial infarction (MI) in the general population [25], severe course of inflammation with arthritis in inflammatory bowel disease [26], vascular involvement in Behçet’s disease (BD) [27].
The hallmark of FMF is a self-limiting febrile attack of polyserositis followed by attack-free period. In a large proportion of patients symptom-free intervals of the disease are characterized by subclinical inflammation with overproduction of C-reactive protein (CRP), serum amyloid A (SAA) and other acute-phase reactants [28,29]. During attack-free periods, patients with FMF may still experience arthritis, myalgia, autonomic dysregulation with altered cardiovascular reactivity [30] and other cardiovascular symptoms related to persistent subclinical inflammation [18]. Importantly, recent studies with cardiovascular imaging techniques identified signs of diastolic dysfunction and microvascular coronary pathology which were correlated with elevated levels of CRP during attack-free period [31, 32]. Clinical significance of coronary pathology in FMF was also highlighted in a few previous reports of coronary vasculitis, transient left bundle branch block and MI associated with inflammation in FMF [33-35].
Persistent inflammation in FMF may cause endothelial dysfunction, atherothrombosis and systemic amyloidosis, all of which share some genetic, immunological and morphological features [36]. Systemic amyloidosis is the most dreadful manifestation of the disease which define its outcome [37, 38]. Rarely, amyloidosis in FMF affects the myocardium and coronary vessels [39-41].
ENDOTHELIAL DYSFUNCTION IN FMF
Endothelial cells play a crucial role in the migration of activated neutrophils into the subendothelial space and in the initiation of the vessel wall inflammation [42]. Endothelial adhesion molecules, such as intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1), facilitate cellular interactions between neutrophils, T- and B-lymphocytes, monocytes/macrophages, endothelial cells, whereas neutrophils, as an abundant source of oxygen reactive species and proteolytic enzymes, act against endothelial cell junctions, thus opening route toward vascular smooth muscle cell [43]. This basic mechanism of endothelial dysfunction initiated by neutrophils underlies diverse disorders associated with inflammation [44, 45] and may play even more active role in FMF.
Though vasculopathic cellular and molecular agents are not fully explored within the frames of FMF, neutrophils are believed to be the main inflammatory cells implicated in endothelial dysfunction, oxidative stress and overproduction of adhesion molecules in this disorder [46]. Neutrophils of patients with FMF may remain hyperactive during attackfree period due to the sustained overproduction of interleukins-17 (IL-17) and -18 (IL-18) [47]. Of note, IL-18 is one of the main proinflammatory cytokines, exerting its proatherogenic effects via inflammasome activation and release of adhesion molecules [48], secretion of interferon-gamma [49] and upregulation of other proinflammatory cytokines (e.g., interleukin-8 [IL-8]) [50].
It has been shown that attack-free period in FMF is characterized by ongoing endothelial damage with elevation of circulating markers of endothelial dysfunction such as thrombomodulin [51], adrenomedullin and nitrite [52]. Elevated CRP [53] and vascular endothelial growth factor [54, 55] can be considered as direct contributors to the vascular damage and endothelial dysfunction in FMF.
PROTHROMBOTIC STATE IN FMF
Over the past few years, several reports have been published, linking subclinical inflammation with markers of hypercoagulation and thrombosis in FMF [56-60]. As a result, it has become possible to formulate hypothesis which considers FMF as a prothrombotic disorder [57]. Interestingly, implications of the MEFV gene in thrombosis have not been investigated in FMF, but its common mutations have been associated with the susceptibility to venous thrombosis in BD [61].
Hypercoagulation in attack-free period in FMF was characterized by shortened prothrombin time and thrombin time, decreased protein C activity and elevation of prothrombin fragment F1+2 [56]. Besides, in one study, platelet activation was documented by an increase of the size of circulating platelets during the same period in FMF [62]. However, in another similar study, there was no difference in platelet function among patients with FMF and healthy controls which was interpreted as a positive (anti-platelet) effect of colchicine therapy [63].
It should be emphasized that platelet activation is an essential link between inflammatory, thrombotic and atherogenic pathways [64]. Activated platelets interact with neutrophils and endothelial cells and through the release of P-selectin and other proinflammatory agents facilitate participation of immune cells in vascular inflammation and atherogenesis [65].
In this regard, further studies are warranted to investigate interaction of platelets with markers of inflammation derived from other cells (e.g., IL-8, neutrophilic enzymes, CRP) and to determine vascular predictive value of activated platelets in FMF.
ATHEROSCLEROTIC BURDEN IN FMF
Surrogate markers of atherosclerosis, such as the carotid artery intima-media thickness (IMT) has emerged as an useful tool for assessment of the burden of atherosclerosis and efficiency of cardiovascular therapies [66]. In most studies, the observed association of circulating inflammatory markers with an increased carotid artery IMT in chronic inflammatory disorders led to a conclusion that these disorders are associated with accelerated atherosclerosis and heightened risk of atherothrombotic events [67].
Atherosclerotic burden measured by the carotid artery IMT was also assessed in patients with FMF [17, 68-71]. Intima-media thickening was associated with elevation of SAA and fibrinogen during attack-free period [17]. None of the studies investigating carotid atherosclerosis in FMF found an increase of the prevalence of atherosclerotic plaques which was seen in high-grade inflammatory disorders (e.g., SLE, RA). The latter can be interpreted as a result of less aggressive course of atherosclerosis in FMF [71]. It is, however, possible to speculate that colchicine therapy was responsible for less aggressive course of atherogenesis in the most studies in FMF. Prospective studies with the patients examined for atherosclerotic burden at baseline (when the diagnosis is made and colchicine administered) and at several time-points of the long-term follow-up, most probably, will ascertain whether colchicine affects the course of atherogenesis in FMF.
COLCHICINE THERAPY AND ATHEROGENESIS
Colchicine is an alkaloid derived from the plant Colchicumautumnale. Extract of the plant has been successfully used for the treatment of gouty arthritis for centuries [72]. Also, colchicine has been demonstrated effectiveness in various inflammatory and (auto)immune disorders (e.g., liver cirrhosis, necrotizing vasculitis, BD, psoriasis, scleroderma, sarcoidosis, thrombocytopenic purpura, pericarditis, skin diseases with neutrophils infiltration) [72, 73]. However, the first report on successful treatment of attacks of FMF with colchicine published in 1972 [74] and large clinical trials [75, 76] distinguished colchicine as a drug of choice for prevention of attacks and amyloidosis in FMF. As a result, nowadays, regular colchicine intake at a dose of 1-2 mg/day is considered as a relatively safe and effective therapeutic option for the suppression of inflammation and prevention of inflammatory co-morbidities in FMF [77].
The main mechanism of action of colchicine, favoring its use in FMF, is dependent on selective accumulation in neutrophils, disruption of polymerization of microtubules, and suppression of neutrophils chemotaxis [78]. Colchicine alters expression of the MEFV [79] and other genes regulating inflammatory pathways [80] exerts anti-cytokinergic and fibrolytic effects [81, 82] further expanding its use in inflammatory disorders (most notably in recurrent pericarditis [83]).
Preliminary data suggest that anti-inflammatory profile of colchicine may have cardiovascular implications in FMF. In a 6-month colchicine trial, patients with FMF at preamyloidal and early amyloidal stages demonstrated significant improvement of diastolic function [84]. In another retrospective study with a large cohort of FMF patients (n=290) regularly treated with colchicine the prevalence of ischemic heart disease (IHD) and established cardiovascular risk factors were comparable to that of healthy controls [85].
Promising results of the studies on anti-inflammatory and cardioprotective effects of colchicine in FMF made it possible to investigate benefits of colchicine in patients with cardiovascular disease with elevated levels of inflammatory markers. In particular, in a one-month study with 44 patients with stable IHD and elevated CRP (>2.0 mg/L), colchicine (1 mg/day) significantly lowered CRP without side effects [86]. Unfortunately, owing to the safety concerns, there are no long-term studies which could clarify whether the observed effect on CRP has implications in terms of reducing vascular events.
Established short-term anti-inflammatory effects and safety profile of colchicine may however have implications for prevention of restenosis in patients undergoing percutaneous transluminal angioplasty. Although clinical evidence to support this hypothesis is non-existent (perhaps due to the safety concerns), one recent experimental study on angioplasty of iliofemoral arteries in dogs demonstrated safety and dose-dependent potential of colchicine in preventing intimal hyperplasia within 2 weeks of the drug therapy [87]. Importantly, further studies in different animal models of angioplasty at coronary and other vascular sites with different regimes of colchicine therapy (oral intake, local application, low, moderate, high doses) are warranted before clinical studies.
CONCLUSIONS
Preliminary studies suggest that attack-free periods of FMF are characterized by subclinical inflammation and associated endothelial dysfunction, increased atherosclerotic burden and platelets activation. Colchicine demonstrated relatively safe anti-inflammatory potential which seems potentially useful for amelioration of endothelial function, suppression of prothrombotic pathways and decrease of atherosclerotic burden (Fig. 1). The effects of colchicine on cardiovascular morbidity and mortality have not been studied prospectively. Nevertheless, clinical experience on regular colchicine therapy accumulated over the past decades suggests that in populations of patients exposed to colchicine from early stages of the disease (e.g., the patients from Israel) the prevalence of IHD is not higher than that in the general population [85]. Moreover, in most previous retrospective studies, those on regular colchicine therapy were free of established cardiovascular risk factors, such as dyslipidemia and hypertension. Given the differences in clinical manifestations, cardiovascular risk profiles and responsiveness to colchicine in populations of FMF patients from different countries, multinational prospective studies with emphasis on established and novel risk factors and vascular events in FMF are recommended.
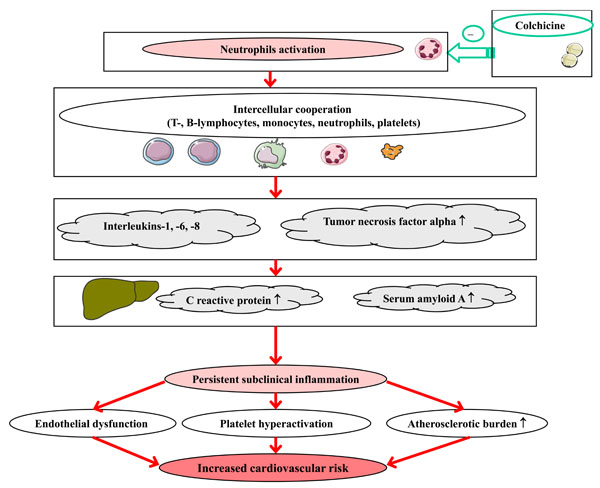
Potential implications of colchicine therapy in atherogensis in FMF.
New opportunities for targeted use of cardiovascular and disease-modifying drugs in FMF and other low-grade inflammatory disorders may emerge with future prospective studies specifically looking at cardiovascular risk profiles in association with several inflammatory (IL-1beta, IL-6, IL-8, TNF-alpha, IL-18) and genetic markers (the MEFV gene mutations).


