All published articles of this journal are available on ScienceDirect.
Feto-Placental Atherosclerotic Lesions in Intrauterine Fetal Demise: Role of Parental Cigarette Smoking
Abstract
The atherogenic effect of cigarette smoking is already recognizable in coronary arteries of fetuses in the last gestational weeks. In this study we analyzed the atherogenic effect of mother’s and father’s smoking habit on coronary arteries and even on adnexa of 30 human fresh fetuses died from 32 to 41 gestational weeks. In 12 cases only the mothers of the victims were cigarette smokers, in 7 cases only the fathers were smokers, whereas in 11 cases nobody smoked.
We observed pre-atherosclerotic and initial atherosclerotic lesions of the adnexa in 21 cases, of which 11 cases had only mother smokers and 6 cases only father smokers. The atherogenic effect is statistically significant in both smoker groups, but stronger in maternal one. The atherosclerotic lesions found in umbilical and placental arteries are similar to those described in fetal coronary arteries: thickening of the arterial walls caused by proliferation and migration of the smooth muscle cells of the tunica media with loss of polarity and infiltration of the subendothelial connective tissue.
INTRODUCTION
In previous works on SIDS and Perinatal Loss [1-3] we observed pre-atherosclerotic lesions in arteries, mainly coronary arteries and conduction system arteries, of fetuses with smoker mothers or exposed to passive smoke [4]. In this study our histopathological investigations on placental arteries and cords showed serious lesions, of unclear nature, related to maternal smoking in pregnancy.
Vascular alterations are common in adnexa. The term “fetal thrombotic vasculopathy” has been used to regroup these alterations, which include fetal artery thrombosis, haemorragic endovasculitis, fibromuscular sclerosis and fibrinous vasculosis [5]. Any factor which adversely influences trophoblastic function restrict the fetal supply of oxygen and nutrients, this resulting in fetal growth retardation, wasting, hypoxia or, in extreme cases, death [6].
The aim of this work was to demonstrate if these alterations are initial atherosclerotic lesions, as the ones described in other fetal arteries. Therefore we hystologically examined cords and placental arteries, together with coronary arteries, of 30 fresh fetuses. The results have been correlated with maternal and paternal smoking, taken separately.
MATERIALS AND METHODS
We studied a sample of 30 cases of fetal death (ranging in age from 32 to 41 weeks of gestation), 17 males and 13 females, weight between 1540 g and 3940 g. The mothers were aged from 22 to 38 years (age average: 30 years old). Nineteen fetuses on 30 had smoking parents (> 6 cigaretttes per day). In 12 cases only the mothers were smokers, in 7 cases only the fathers were smokers, whereas in 11 cases nobody smoked (Table 1).
Case Profiles and Results of the Study
| Case n | Sex | Gestational Age (Weeks) | Body Weight (g) | Parental Smoking | Pre-Atherosclerotic Lesions | ||
|---|---|---|---|---|---|---|---|
| Coronary | Cords | Placenta | |||||
| 1 | M | 39 | 3940 | p | + | + | + |
| 2 | F | 38 | 3530 | p | + | + | + |
| 3 | F | 37 | 2880 | m | + | + | + |
| 4 | F | 40 | 3340 | no | + | + | + |
| 5 | F | 34 | 1870 | no | + | + | + |
| 6 | M | 41 | 3470 | p | + | + | + |
| 7 | M | 32 | 1700 | p | - | + | - |
| 8 | M | 39 | 3700 | no | - | + | + |
| 9 | F | 38 | 3200 | m | + | + | + |
| 10 | M | 39 | 3580 | no | - | - | - |
| 11 | M | 39 | 2050 | m | + | - | - |
| 12 | F | 40 | 3100 | m | + | + | + |
| 13 | M | 40 | 3200 | p | + | + | + |
| 14 | F | 36 | 2200 | no | - | - | - |
| 15 | F | 38 | 3530 | no | - | - | - |
| 16 | F | 32 | 1680 | m | - | + | + |
| 17 | M | 36 | 2200 | no | - | - | - |
| 18 | F | 36 | 1840 | m | + | + | + |
| 19 | M | 34 | 2350 | no | - | - | - |
| 20 | M | 38 | 3160 | m | + | + | + |
| 21 | M | 39 | 2330 | m | + | + | + |
| 22 | M | 38 | 3530 | m | + | + | + |
| 23 | F | 33 | 1840 | no | - | - | - |
| 24 | M | 32 | 1540 | p | - | + | + |
| 25 | M | 38 | 3210 | p | + | + | + |
| 26 | M | 39 | 3250 | m | + | + | + |
| 27 | F | 40 | 3060 | no | - | - | - |
| 28 | M | 35 | 2480 | m | + | + | + |
| 29 | M | 36 | 2650 | m | + | + | + |
| 30 | F | 40 | 3100 | no | - | - | - |
M: male
F: female
m: maternal smoking
p: paternal smoking
no: no smoking
+: lesion
-: no lesion
Studying the clinical information about the single pregnancy of the victim’s mothers we found that in each case pregnancy had run a normal course and the mothers had normal plasma cholesterol levels (total plasma cholesterol ≤ 185-200 mg/dl), and no diabetes or bleeding alterations. Regarding the use of medicaments during the pregnancy, only 1 mother had assumed anxiolytic for few times. Only one mother had a spontaneous abortion in the past.
A complete autopsy examination was carried out including a systemic gross and microscopic evaluation of the body, the placental disk, the umbilical cord and membranes. All organs were fixed in 10% phosphate-buffered formalin, processed and embedded in paraffin. Sections (5 µm) were stained with hematoxylin and eosin.
Coronary Artery Examination
Following the protocol of Roberts et al. [7], the hearths were fixed in formalin for at least 1 day. The four major epicardial coronary arteries (left main, left anterior descending, left circumflex, and right) were excised transversely to their longitudinal axis into segments 2-3 mm long. They were dehydrated, embedded in paraffin, and serially cut. The sections of each block were stained with hematoxylin and eosin, and Heidenhain’s trichrome (Azan).
Adnexa Artery Examination
Three segments of umbilical cord were excised, fixed in 10% phosphate-buffered formalin, processed and embedded in paraffin. Regarding the placenta, a cotyledon macroscopically regular and also the areas considered pathologic or suspect where completely excised, from the decidual plate (maternal side) to the amniotic plate (fetal side), and fixed. Sections (5 µm) were stained with hematoxylin and eosin, and Heidenhain’s trichrome (Azan).
Statistical Analysis
JMP software has been used. Contingency table and chi2 test have been performed. Significance was assumed when p<0,05.
Definition of Terms
Pre-atherosclerotic Lesions
The earliest morphologically recognizable alterations are characterized by fragmentation of the tunica media with intense infiltration of SMCs, often arranged perpendicularly in columns or tangentially to the axis of the tunica itself. These SMCs infiltrate the intima, wich tends to be thickened (2, 3).
Initial Atherosclerotic Lesions
These lesions are characterized by wide myointimal thickeness with alteration of elastic fibers and of the tunica media, also due to initial mucopolysaccharide deposits, mainly composed of tipe A and C chondroitin sulfates and hyaluronic acid. Rare moncytes and B-lymphocytes are also present. The endotelium is morphologically intact. The internal elastic membrane appears to be focally fragmented (2, 3).
RESULTS
We evaluated the histological pattern of coronary, umbilical and placental arteries of all the 30 victims of the study. Afterward we correlated the results obtained with parental smoking.
Coronary Arteries
We observed a regular structure of the arterial wall in 12 cases. In the other 18 cases, all aged over 34 gestational weeks, we found multifocal alterations of all coronaries: variable thickening of the wall, distortion of the normal wall’s architecture and pre-atherosclerotic lesions. The muscular fibrocells were fragmented into little cylindrical segments that, loosing the cellular polarity, formed columns of myocytes located perpendicularly or tangentially to the axis of the media itself, gradually infiltrating the intima. (Figs. 1 and 2). The endothelium appeared in all cases intact.
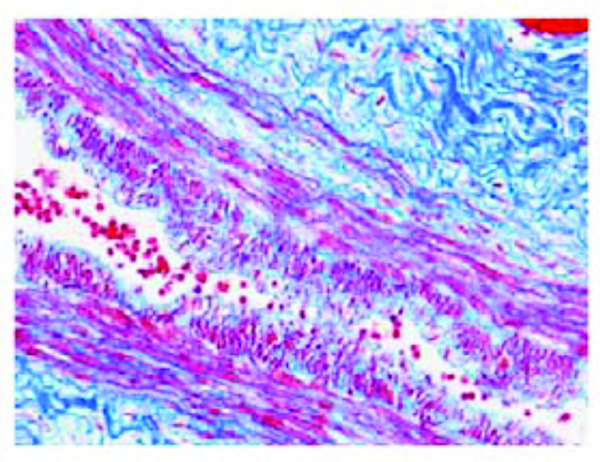
Pre-atherosclerotic lesion in an epicardial coronary artery (left anterior descending branch) of a fetus aged 40 weeks (case n.12). The focal thickness consists of medial smooth muscle cells, perpendicularly oriented and infiltrating the subendothelial connective tissue. Azan stain. Magnification: 20x.
Initial atherosclerotic lesion in an epicardial coronary artery (left anterior descending branch) of a fetus aged 38 weeks (case n. 2). Two opposed early atherosclerotic plaques are visible, markedly narrowing the coronary lumen. Azan stain. Magnification 10x.
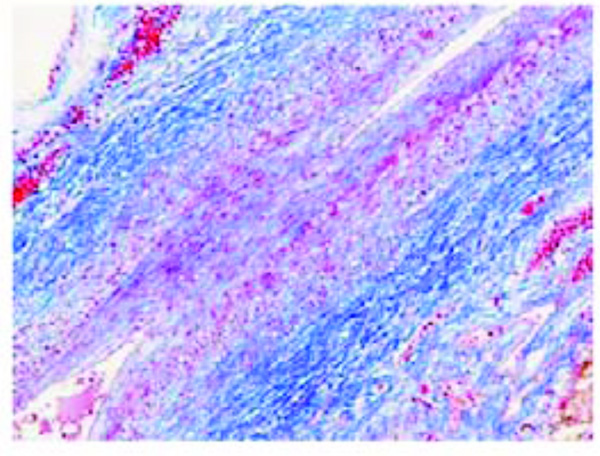
Initial atherosclerotic lesion in an umbilical artery of a fetus aged 39 weeks (case n. 21). The myo-intimal thickness shows numerous smooth muscle cells with loss of polarity and increased amounts of mucoid ground substance. Azan stain. Magnification: on the left 20x; on the right 40x.
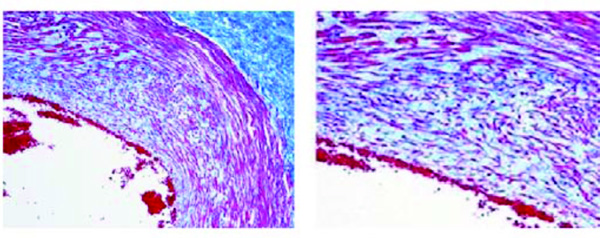
Initial atherosclerotic lesion in a villi artery of a fetus aged 40 weeks (case n. 13). The thickness shows marked alteration of the muscular tunica with thinning, fiber fragmentation and diffuse infiltration of the intima. Azan stain. Magnification: on the left 10x; on the right 20x.
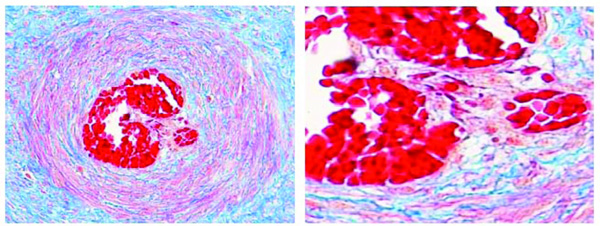
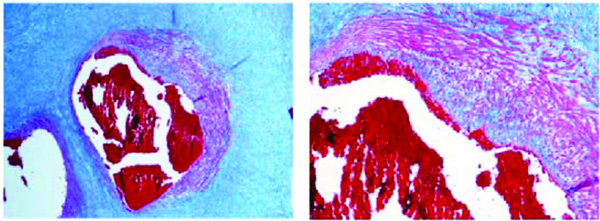
Artery in a stem villus of a fetus aged 36 weeks (case n. 18). Proliferating smooth muscle cells protrude into the lumen producing septations of the lumen itself. Azan stain. Magnification: on the left 10x; on the right 20x.
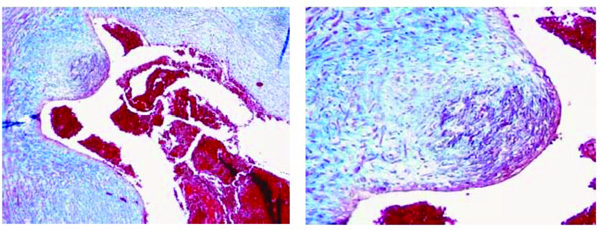
Initial atherosclerotic lesion in a villi artery of a fetus aged 40 weeks (case n. 12). The myo-intimal thickness is characterized by a wide-spread presence of smooth muscle cells associated with a moderate presence of monocytes/foam-cells. Azan stain. Magnification: on the left 10x; on the right 20x.
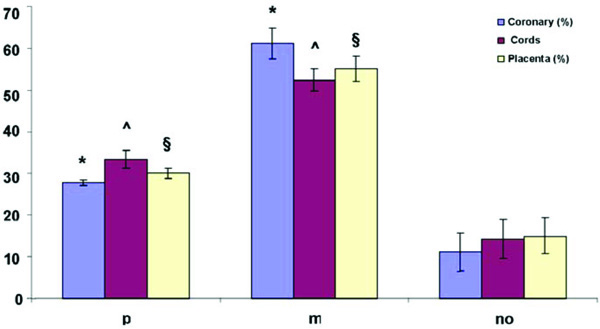
*p<0.05 Coronary group p, Coronary group m vs Coronary group no.
ˆp<0.05 Cords group p, Cords group m vs Cords group no.
§p<0.05 Placenta group p, Placenta group m vs Placenta group no.
Umbilical Arteries
The histological study of the cords showed structural alterations of the umbilical arteries superimposable or more severe than the lesions found in the coronary arteries. In 21 cases we found remarkable initial atherosclerotic lesions characterized by myointimal thickening with smooth muscle cells proliferation, disposed perpendicularly or tangentially to the axis of the media itself, and increased amounts of mucoid substance (Fig. 3).
Placenta
The placentas were all single, weight between 300 g and 860 g. The microscopic report showed in each case villi according to gestational age (III trimester) and normally arborized. In 4 cases we found old infarctions and in 1 case hypercapilarization of the term villi. Paying attention on the arterial vessels examination, we focused on chorial arteries, localized in the fetal side of the placenta, and arteries of the villi (coming from umbilical arteries); especially stem villi and intermediate villi.
The wall artery layers showed structural alterations (pre-atherosclerotic lesions and initial atherosclerotic lesions) in 20 cases on 30, similar to those already found during the histologic study of the other arterial vessels. In stem villi arteries of some placental sections, we observed different structural alterations: thickening caused by proliferation and migration of the smooth muscle cells with changing of the normal structure, characterized by circular and concentric bundles of the tunica media; muscular cells protruding into the lumen and in some vessels even real septations of the lumen itself; partial structure of the muscular tunica, replaced by fibro collagen tissue; loss of polarity, forming columns of myocites located perpendicularly to the axis of the media itself and infiltrating the subendothelial connective tissue; intimal pre-atherosclerotic lesions of proliferative aspect, characterized by a widespread presence of smooth muscle cells; fragmentation of the elastic fibers and of the tunica media, that appears focally thinner (Figs. 4, 5 and 6).
Correlation with Parental Cigarette Smoking
We compared p and m groups (p: paternal smoking; m: maternal smoking) with no smoking group.
In 19/30 fetuses we found a strong correlation between parental smoking and pre-atherosclerotic lesions in the examined arteries (Graphic 1). We also demonstrated the different and significant impact of maternal smoking on the fetus than paternal. Only in three cases with no smoking parents (n.4, 5 and 8) pre-atherosclerotic lesions were observed.
DISCUSSION
Atherosclerosis is a dynamic process marked by the strong reactivity of the myofibroblast making up the muscular tunica that is responsible for defending the vessels against external atherogenic noxae [8, 9]. Myofibroblast proliferation and migration to the intima is the sine qua non of onset of the atherosclerotic process. The trophism of the internal portion of the arterial wall, the intima and most of the media, is based on filtration of the metabolites deriving from the blood circulation. When the myointimal architecture is altered, due to structural changes or excessive deposits of metabolites, this filtration process is impeded, which in turn contributes to further deposition of metabolites [10]. The artery wall is almost lacking in defensive cells. The only cells that are able to react to the action of harmful agents are the smooth muscle cells (SMCs). The fundamental functional activity of these cells in physiological conditions is contractility, which is essential to maintain the tone of the arterial wall. In the presence of harmful agents these cells de-differentiate, reverting to the activities of the mesenchymal cells from which they derive, in other words ameboid movement and phagocytosis. They are also the only cells deputed to the activities of synthesis and secretion of elastic proteins and collagen [11]. In addiction, the SMCs are transformed from the contractile or quiescent phenotype, characterized by the presence of alpha-actin, into the synthetic or activated type, showing loss of the contractile function and a switch in actin expression. Gabbiani et al., have defined these dedeifferentiated cells as “myofibroblasts”, having features in common with both SMCs and fibroblasts [12, 13].
These results indicate that these changes are of an initial atherosclerotic nature, similar to those already highlighted in coronary arteries, caused by the effects of gaseous components released during the combustion of nicotine. Asmussen highlighted similar changes about 30 years ago in umbilical arteries of fetuses of smoking mothers [14, 15].
In this work we studied 30 unexpected and unexplained fetal deaths, in which the fetal, umbilical and placental arterials system showed initial atherosclerotic lesions not only in arteries of fetal organs, above all coronaries, but also in umbilical and placental arteries. These alterations are consistent with those found in the coronary arteries and they are characterized by a focal deep subversion of the tunica media with fragmentation of the smooth muscle cells, loss of polarity and infiltration of the subendothelial connective tissue of the intima. These alterations, which are often more important than those described in the coronary arteries, can reduce the placental flow and oxygenation. Therefore in this situation the placenta can’t offset the physiological hypoxemic condition of the fetus. In fact, the situation in the fetus before birth is quite similar to a 2 minutes period of hypoxic apnea in the adult animals, during which time the circulation is manteined with bradicardia and hypertension (a situation reminiscent of the dive reflex), except that placental circulation permits stabilization of the blood gases at that level [16]. Fetal hypoxaemia may result from altered maternal PaO2 or a reduction in maternal placental or umbilical blood flow [17]. Adherence to this belief implies that any factor which adversely influences trophoblastic function will restrict the fetal supply of oxygen and nutrients, this resulting in fetal growth retardation, wasting, hypoxia or, in extreme cases, death [6].
In healthy smoker mothers with a normal lipid status, the fetus is an ideal model in which to study the effects of passive smoke on the arterial wall [10]. In fact, as we demonstrated in previous works, the gaseous products of nicotine combustion represent pathogen noxa, which cause a severe morphological alteration of the tunica media of the arterial vessels [10]. In case of maternal smoking in pregnancy, nicotine is easily crossing the placenta exposing the fetus to a higher nicotine concentration than the smoking mother. Nicotine reduces fetal oxygenation through increased blood levels of carboxyhemoglobin and through impairment of oxygen unloading and also causes vasoconstriction which, along with the reduced prostacyclin synthesis, increases vascular resistance and decreases fetal blood flow.
Exposure to smoking in utero may thus cause damage to the developing organs, especially that most susceptible to hypoxic damage, including the brain and the annexa itself [18-20]. Smoking can also exert a direct effect in fetal arterial walls, causing atherosclerotic alterations, which may be responsible on fetal hypoxia itself and it represents a causal factor for unexpected and unexplained fetal deaths [1, 2].
Environmental tobacco smoke, called “secondhand smoke” (SHS) is known to increase morbidity and mortality risks in infants, children and adults non-smokers [21, 22]. The 2006 Surgeon General’s report on involuntary smoking concluded that more than 126 million of people are exposed to SHS, 50.000 deaths per year are caused by SHS and it’s important to stress that there is no “safe level” of exposure [23]. A recent study of Winickoff et al., introduces a new concept, the “thirdhand” smoke that represent the residual tobacco smoke contamination that remains after the cigarette is extinguished in every surface [24]. Infants and children are especially subjected to thirdhand smoke exposure because they often are in contact through hands and mouth to contaminated surfaces. Also the future mothers in pregnancy are exposed to this “passive smoke” (probably because the husband or anyone else smoke).
In particular, our research into the harmful effects of cigarette smoking on the fetal arterial wall has contributed to a better definition of the morpho-functional pictures at onset and progression of atherosclerosis, since the reactive properties of the SMCs are particularly string in fetal arteries [11]. Pre-atherosclerotic lesions, as we demonstrated in this work, are already present in the arteries of fetuses from the 32nd week of gestation with smokers mothers and these alterations are characterized by marked structural changing of the tunica media that affect first the inner portion, luminal, and then completely the thickness of the wall, probably because of the progressive diffusion of the almost gaseous agents deriving from the blood circulation [1].
The results obtained from the histologic study of umbilical and placental arteries, led us to demonstrate the incidence of pre-atherosclerotic lesions, their characteristics and the possible role of maternal and/paternal smoking in their pathogenesis.
Probably, air pollution is the cause of the lesions observed in the threee cases of this study with no smoking parents.
ACKNOWLEDGEMENTS
The authors thank Prof. Maria Elena Ferrero, MD, and Mr. Alessandro Fulgenzi for helping with statistical analysis.


