All published articles of this journal are available on ScienceDirect.
Implementation of Guidelines for the Management of Arterial Hypertension. The Impulsion Study
Abstract
This study assessed the effects of a pilot best practice implementation enhancement program on the control of hypertension. We enrolled 697 consecutive known hypertensive patients with other vascular risk factors but free from overt vascular disease. There was no “control” group because it was considered unethical to deprive high-risk patients from “best medical treatment”. Following a baseline visit, previously trained physicians aimed to improve adherence to lifestyle measures and drug treatment for hypertension and other vascular risk factors. Both at baseline and at study completion (after 6 months), a 1-page form was completed showing if patients achieved treatment targets. If not, the reasons why were recorded. This program enhanced compliance with lifestyle measures and increased the use of evidence-based medication. There was a substantial increase in the number of patients who achieved treatment targets for blood pressure (p<0.0001) and other vascular risk factors. In non-diabetic patients (n=585), estimated vascular risk (PROCAM risk engine) was significantly reduced by 41% (p<0.0001). There was also a 12% reduction in vascular risk according to the Framingham risk engine but this did not achieve significance (p=0.07). In conclusion, this is the first study to increase adherence to multiple interventions in hypertensive patients on an outpatient basis, both in primary care and teaching hospitals. Simple, relatively low cost measures (e.g. educating physicians and patients, distributing printed guidelines/brochures and completing a 1-page form) motivated both physicians and patients to achieve multiple treatment goals. Further work is needed to establish if the improvement observed is sustained. [ClinicalTrials.gov NCT00416611].
INTRODUCTION
Cardiovascular disease (CVD) morbidity and mortality show a continuous relationship with both systolic and diastolic blood pressure (BP) levels, without any evidence of a threshold down to at least 115/75 mmHg [1]. Hypertension is considered a major modifiable risk factor for stroke and also increases the risk for coronary heart disease (CHD), heart failure, chronic kidney disease (CKD) and peripheral arterial disease [2]. Randomized placebo-controlled trials showed that BP lowering is associated with a reduction in the risk of fatal and non-fatal stroke (by about 30-40%), while CHD events are reduced to a lesser extent (by about 20%) [3-8]. Comparative studies showed that for a similar BP lowering, differences in vascular risk reduction between various drug classes are small [7, 9].
According to the National Health and Nutrition Examination Survey (NHANES) data, the prevalence of hypertension increased significantly between 1988 and 2004 [10]. Between 1988 to 1994 and 1999 to 2004, BP control increased in men from 39% to 51% (p<0.05) but did not change significantly in women (35% to 37%) [10]. Control rates were lower in persons older than 70 years and in patients with type 2 diabetes mellitus (T2DM) or CKD [10]. Similar hypertension control rates were recently reported in Europe [11].
In the present study we assessed whether a best practice implementation enhancement program could improve control of hypertension and other vascular risk factors in hypertensive patients without clinically overt vascular disease.
STUDY DESIGN AND METHODS
The IMPULSION (Implementation of guidelines for the management of arterial hypertension). Study was conducted during 2006-2007 under the auspices of the Regional (Bureau) Authority of the Ministry of Health for Northern Greece-Central Macedonia. The Working Groups for the identification and treatment of hypertension of the Hellenic Atherosclerosis Society and the Greek Society of General Practitioners jointly conducted the study. The study received ethical approval and informed consent was obtained from all patients before enrolment. This study is registered as ClinicalTrials.gov NCT00416611.
Definition of Hypertension, Dyslipidemia, T2DM and Metabolic Syndrome (MetS)
At baseline, BP was measured in both arms by a trained physician using a standard mercury sphygmomanometer after 5 min rest with the subject in the sitting position. The arm with the highest reading was selected [2, 12]. The mean of 2 measurements, taken 1 min apart, was recorded in the selected arm. The disappearance of Korotkoff sound (onset of phase 5) was used to define diastolic BP (DBP). All patients were on treatment with antihypertensive medication [13]. Hypertension was considered to be controlled if the BP was ≤140/90 mmHg. In patients with T2DM, BP was considered to be controlled when it was ≤130/80 mmHg [13].
Patients were considered to have dyslipidemia when: i) low density lipoprotein cholesterol (LDL-C) levels were <160 mg/dl (4.2 mmol/l), <130 mg/dl (3.4 mmol/l), <100 mg/dl (2.6 mmol/l) or <70 mg/dl (1.8 mmol/l) in lower risk, moderate/moderately high risk, high risk (CHD or CHD risk equivalents) or very high risk (CHD with T2DM or MetS or smoking) patients, respectively [14], ii) high density lipoprotein cholesterol (HDL-C) levels were < 40 mg/dl (1.0 mmol/l) in both genders [15], iii) non-HDL-C levels were >30 mg/dl higher than the LDL-C target levels (according to risk status) in patients with triglyceride (TG) levels >200 mg/dl (2.2 mmol/l) [14], or, iv) patients were on lipid-lowering treatment. Only patients with TG levels <400 mg/dl (4.5 mmol/l) were included in the study, since the Friedewald formula [LDL-C = total cholesterol - (TG / 5 + HDL-C) in mg/dl] was used to calculate LDL-C levels.
According to the American Diabetes Association criteria, patients were considered to have T2DM when the fasting venous plasma glucose levels were ≥126 mg/dl (7.0 mmol/l) on 2 consecutive assessments or if they were on treatment for T2DM [16].
Participants with 3 or more of the following criteria [according to the National Cholesterol Educational Program Adult Treatment Panel III (NCEP ATP III) report] [17] were considered to have MetS:
- Waist circumference (WC) >102 cm in men and >88 cm in women
- Fasting TG ≥150 mg/dl (1.7 mmol/l) or on treatment for TG
- HDL-C <40mg/dl (1.0 mmol/l) in men and <50 mg/dl (1.3 mmol/l) in women or on treatment for HDL-C
- BP ≥130/85 mmHg or use of antihypertensive medication
- Fasting venous plasma glucose≥110 mg/dl (6.1 mmol/l) or treatment
Study Design - Study Cohort
This was a best practice prospective study. Physicians from Health Centres (primary care) or Hospitals (secondary care) recruited consecutive consenting patients with known hypertension, with or without other vascular risk factors, but free of overt CVD at baseline. A total of 697 hypertensive patients were enrolled. Seven additional patients that were planning to move to a region outside the program area did not agree to participate. These 7 patients were not different in their demographic or clinical characteristics from those who agreed to participate. Besides, their number is so small that it could not have changed the baseline status or final outcome. Apart from these 7 patients, no other patient refused to participate.
During the first visit, personal and family history, as well as medication, was recorded in a specially designed 1-page form (Table 1). A physical examination was also carried out. Afterwards, laboratory tests were performed at the hospital following a 12h fast, at which time a second physical examination was performed to confirm the clinical findings.
Patient Data Form
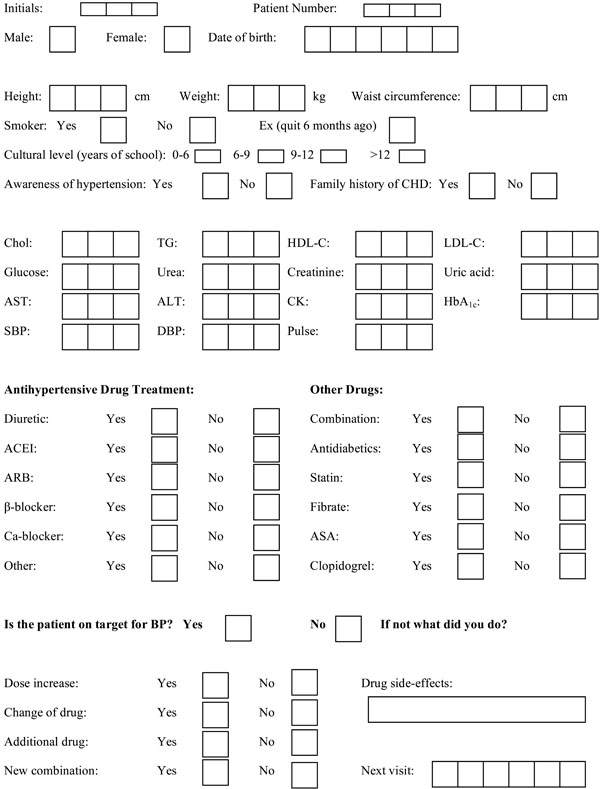 |
Patient Characteristics at Baseline and at the End of the Study (i.e. After 6 Months)
| Baseline n=697 | End of Study n=697 | P | |
|---|---|---|---|
| Age (years ± SD) | 59±8 | ||
| Men (%) | 38.7 | ||
|
Years of education (%) <6 6-9 10-12 University education |
54.6 24.8 13.7 6.9 |
||
| Body weight (kg) | 82±12 | 79±13 | NS |
| Waist circumference (cm) | 97±13 | 96±9 | NS |
| Smoking (%) | 33.6 | 32.2 | NS |
| Systolic BP (mmHg) | 154±19 | 136±13 | <0.0001 |
| Diastolic BP (mmHg) | 92±11 | 83±7 | <0.0001 |
| At systolic BP target (%) | 21.8 | 54.7 | <0.0001 |
| At diastolic BP target (%) | 32.7 | 64.2 | <0.0001 |
| At both systolic and diastolic BP target (%) | 19.3 | 49.2 | <0.0001 |
| Dyslipidemia (%) | 59.7 | 53.6 | NS |
| Total cholesterol (mg/dl) | 223±36 | 205±28 | <0.0004 |
| Triglycerides (mg/dl) | 153±44 | 134±32 | <0.002 |
| LDL-C (mg/dl) | 143±31 | 125±23 | <0.0001 |
| HDL-C (mg/dl) | 44±13 | 45±14 | NS |
| On lipid targets (in the whole population, n=697) (%) | 44.6 | 72.8 | <0.0001 |
| On lipid targets (in patients on statins) (%) | 17.2 (n=173) | 68.8 (n=273) | <0.0001 |
| Type 2 diabetes mellitus (%) | 16 | 14.8 | NS |
| Fasting plasma glucose (mg/dl) | 106±32 | 101±22 | <0.005 |
| HbA1c(%)(in diabetic patients, n=112) | 7.4±0.5 | 6.1±0.6 | <0.0001 |
| MetS (men) | 37.8 | 29.3 | <0.0001 |
| MetS (women) | 45.9 | 34.2 | <0.0001 |
| Serum creatinine (mg/dl) | 0.90±0.34 | 0.89±0.27 | NS |
| Serum uric acid (mg/dl) | 5.5±2.4 | 5.3±2.6 | NS |
| AST (IU/l) | 24±9 | 23±8 | NS |
| ALT (IU/l) | 25±11 | 24±9 | NS |
| 10-year patient risk PROCAM % (in non-diabetic patients, n=585) | 11.2 | 6.6 (-41%) | <0.0001 |
| 10-year patient risk Framingham % (in non-diabetic patients, n=585) | 12.2 | 10.7 (-12%) | NS |
BP, blood pressure; LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; HbA1c, glycated hemoglobin; AST, aspartate transaminase; ALT, alanine transaminase; PROCAM, prospective CV münster study; MetS, metabolic syndrome; NS, not significant.
Drug Treatment at Baseline and at the End of the Study (i.e. After 6 Months)
| Baseline n=697 | End of Study n=697 | p | |
|---|---|---|---|
| Diuretics | 34.5 | 48.7 | <0.0001 |
| ACEIs | 30.3 | 49.6 | <0.0001 |
| ARBs | 29.6 | 45.2 | <0.0001 |
| Beta-blockers | 24.3 | 31.7 | NS |
| Calcium antagonists | 29.8 | 43.5 | <0.0001 |
| Fixed combinations | 20.3 | 37.2 | <0.0001 |
| Antidiabetic drugs | 13.6 | 14.2 | NS |
| Statins | 24.8 | 39.2 | <0.0001 |
| Fibrates | 1.3 | 1.8 | NS |
| Aspirin | 12.6 | 18.5 | <0.0001 |
All numbers express percent of patients.
ACEIs, angiotensin converting enzyme inhibitors; ARBs, angiotensin receptor blockers; NS, not significant.
Intervention
Before study initiation and during the study physicians attended an educational and problem solving program. In all there were 7 meetings. The educational components were presentation of current guidelines for hypertension, dyslipidemia, T2DM, MetS and nutrition (specific diets based on a Greek variation of the Mediterranean diet). Up to date treatment protocols were also discussed. This took 4 meetings. The other 3 meetings were for technical problem solving during the study. To facilitate physician education we supplied current guidelines for the treatment of hypertension and other risk factors and an information brochure for the patients providing advice on lifestyle measures. All these documents (Patient Data Form, Guidelines for the Physician, Patient Brochure) were written using a computer and photocopies were distributed. This was deemed to be a low cost procedure. The physicians agreed to participate as part of their continuing medical education program. They also wanted to have more patients at goal for CVD risk factors.
During the study, an intensification of treatment in terms of lifestyle advice, new drugs or titration of already prescribed drugs was implemented aiming to reach multiple treatment targets. Physicians also advised each patient to improve compliance with a healthier lifestyle and drug treatment. The lifestyle changes included smoking cessation, weight reduction, increased physical activity, decreased salt intake, moderate alcohol consumption and a Mediterranean diet. The patients were advised as to how often they should consume non-refined cereals (e.g. whole grain bread, pasta and rice), potatoes, fruits, vegetables, legumes, fish, red meat and products, poultry, full fat dairy products (cheese, yoghurt and milk), olive oil in cooking and alcoholic beverages [18]. Compliance to the Mediterranean diet was assessed using a 10-unit scale.
Follow-up
In Greece monthly visits are required by health care providers for the renewal (refill) of prescriptions by general practitioners or outpatient clinic physicians. Consequently, patients had to visit their physician every month. At the 6th treatment month (the final visit) physicians completed the same form for each patient that included 2 more questions. Does the patient still have hypertension? And if yes, what did the physician do about it? Physicians were aware of this question from visit 1 and we believe that this was a motivational factor.
There was no “control” group because all patients had hypertension, as well as other vascular risk factors. It was therefore deemed unethical to deprive them of “best medical treatment”. Any changes at the final visit were compared with baseline values. The estimated vascular risk was 11.2 and 12.2% at baseline in non-diabetic patients [assessed with the PROspective CV Münster (PROCAM) and Framingham risk engine, respectively; Table 2] but this was calculated using “on treatment” values. In this context, all patients were taking at least 1 antihypertensive drug and 24.8% of the whole study population were on statins. Furthermore, 16% had T2DM (a CHD risk equivalent [14, 17]) and about 43% had MetS.
Laboratory-Based Assessment
After an overnight 12h fast, serum total cholesterol, HDL-C, TG, transaminases, creatinine and uric acid levels and plasma glucose levels were measured using an Olympus AU 560 autoanalyser (Medicon Hellas, Athens, Greece) and respective reagents (Olympus Diagnostica GmbH, Clare, Ireland). LDL-C was calculated using the Friedewald formula. Glycated hemoglobin (HbA1c) was determined in diabetic patients with high performance liquid chromatography (HPLC) using the Menarini Diagnostics (Menarini, Greece) reagents (reference range: 4.2-6.5%). All hospital laboratories participate in internal and external quality control schemes.
PROCAM and Framingham 10-Year Risk Estimates
The PROCAM Trial risk engine [http://www.chd-taskforce.com] calculates the 10-year risk for fatal or non-fatal myocardial infarction (MI), taking into consideration age, LDL-C, HDL-C, TG, smoking habit (including ex-smokers), fasting blood glucose, systolic BP (SBP), current antihypertensive treatment and family history of premature CVD.
The Framingham risk engine [http://www.chd-taskforce.com] does not consider impaired fasting glucose, ex-smoking, high TG levels and family history of premature CVD when calculating the 10-year risk. Moreover, when the 10-year risk is >30% the program does not provide an exact number.
Since we are comparing pre- with post-intervention calculated risk we allowed an additional ± 5 years of age beyond the limits set by the PROCAM risk engine. This procedure allowed us to compare a greater number of patients.
Statistical Analysis
Analyses were carried out using the SPSS 12.0 software (SPSS Inc., Chicago, IL). All variables had a normal distribution (assessed with the Kolmogorov-Smirnov test) and are reported as mean and standard deviation (SD). The paired samples t-test and Chi-square test were used to evaluate changes in continuous and categorical variables, respectively. A two tailed p <0.05 was considered significant.
RESULTS
Baseline Data
The baseline characteristics of the 697 hypertensive patients recruited in the study are shown in Tables 2 and 3. Twenty seven additional patients attended the baseline visit but did not attend the final visit (after 6 months) and are not included in the final analysis. These 27 patients represent 3.9% of the recruited population.
As already mentioned, all patients were known hypertensives and were receiving at least 1 antihypertensive drug; the mean number of antihypertensive drugs per patient was 1.4. At baseline, 21.8% of patients were on target for SBP (140 mmHg), 32.7% for DBP (90 mmHg) and 19.3% for both SBP and DBP (add to Table 2). Although the prevalence of dyslipidemia was 59.7%, only 24.8% of patients were receiving statins. At baseline, 44.6% of the whole population was at their lipid targets. Among patients on statins, 17.2% were at their target. At baseline, 12.6% of patients were taking aspirin (ASA).
Treatment and Control of Hypertension and Other Vascular Risk Factors
As shown in Tables 2 and 3, the vascular risk factor profile improved significantly during the 6-month follow-up associated with a greater compliance to lifestyle advice (there was a 2-unit increase in adherence to the recommended diet) and a more aggressive pharmacological intervention.
At the end of the study 54.7% of the patients were on target for SBP, 64.2% for DBP and 49.2% for both SBP and DBP (p<0.0001 for all comparisons vs. baseline). Compared with BP at baseline, average SBP was reduced by 18 mmHg (p<0.0001) and DBP by 9 mmHg (p<0.0001). The mean number of antihypertensive drugs per patient increased from 1.4 to 2.8 (p<0.0001) and the use of fixed combinations of antihypertensive drugs increased from 20.3% to 37.2% (p<0.0001).
The prevalence of dyslipidemia at the end of the study decreased by 10.2% (53.6% vs. 59.7% at baseline, p=0.12). There was a significant 58.1% increase in the percentage of patients on statins (from 24.8% to 39.2%, p<0.0001). At the end of the study, 72.8% of the whole population was at their lipid targets (p<0.0001 compared with baseline). Moreover, 68.8% of patients on statins achieved their targets (p<0.0001 compared with baseline).
There was a significant improvement in the glycemic control in diabetic patients; HbA1c decreased by 18% (6.1% vs. 7.4% at baseline, p<0.0001). The prevalence of T2DM at the end of study decreased by 7.5% (14.8% vs. 16% at baseline, p=0.09). At the end of the study, 14.2% of patients were on antidiabetic drugs and 0.6% were only on diet.
There was an increase in the use of ASA by 47% (18.5% vs. 12.6% at baseline, p<0.0001).
There was a reduction in the incidence of MetS by 23% in men (29.3% vs. 37.8% at baseline, p<0.0001) and by 26% in women (34.2% vs. 45.9% at baseline, p<0.0001).
Framingham and PROCAM 10-Year CVD Risk Estimates
In patients without T2DM (n=585, 84% of the study population), the estimated 10-year CHD risk according to the PROCAM risk engine was significantly reduced by 41% (6.6% vs. 11.2% at baseline, p<0.0001). The estimated 10-year risk according to the Framingham risk engine in the same population was reduced by 12% (10.7% vs. 12.2% at baseline, p=0.07). We did not estimate the vascular risk in patients with T2DM (n=112, 16% of the study population) because T2DM is considered a CHD risk equivalent (estimated 10-year risk > 20%).
Safety and Tolerability
Medication was well tolerated and the only reported adverse effects that occurred in more than 2% of the participants were dry cough in users of angiotensin converting enzyme inhibitors (mainly) and of angiotensin receptor blockers (less frequently), as well as ankle oedema in users of calcium antagonists.
DISCUSSION
Our pilot program was effective at implementing multifactorial evidence-based treatment in hypertensive patients, with or without other vascular risk factors, and free of CVD at baseline. Lifestyle measures and aggressive pharmacological treatment was associated with a significant increase in the proportion of patients reaching target for SBP (from 21.8% to 54.7%), for DBP (from 32.7% to 64.2%) and for both SBP and DBP (from 19.3% to 49.2%) (p<0.0001 for all comparisons). There was also a 41% reduction in estimated 10-year CHD risk according to the PROCAM risk engine in non-diabetic patients (from 11.2% to 6.6%; p<0.0001). In the same population, estimated CHD risk according to the Framingham risk engine also decreased by 12% but this fall did not achieve significance (p=0.07). Thus, it is possible that engines incorporating several additional variables (e.g. PROCAM) capture the vascular risk reduction achieved by a multifactorial approach in a more obvious manner than other calculators that consider fewer factors (e.g. Framingham). Whether this means that new broad based risk calculators are required to accurately assess multifactorial approaches to vascular risk reduction remains to be established.
The present study is part of 4 pilot best practice implementation enhancement programs aimed at improving risk factor control in 4 different populations of patients with: (i) hypertension (the present study), (ii) dyslipidemia [Hatzitolios et al, unpublished observations], (iii) MetS [19], or, (iv) T2DM [Athyros et al, unpublished observations]. These programs were similarly effective.
Large surveys in the US, Canada and Europe (Germany, Sweden, England, Spain and Italy) showed that only 29%, 17% and 10% of hypertensive patients, respectively, had BP levels less than 140/90 mmHg [20]. These findings might be explained by the more aggressive guidelines for hypertension treatment in the US compared with Europe. A recent cross-sectional analysis of the 2004 data from CardioMonitor, an ongoing survey in ambulatory patients with CVD in the US and in 5 European countries (Germany, France, England, Spain and Italy) showed an improvement in control rates [11]. The rate of hypertension control (<140/90 mmHg) was 63% in US and 31-46% in Europe [11]. Several studies evaluated hypertension control rates in Greece [21-25]. In 2001, the Greek component of the European Prospective Investigation into Cancer and nutrition (EPIC) study (26, 913 volunteers from several regions of Greece) showed that BP was controlled only in 15.2% of hypertensive patients [21]. In the Attica study (carried out between 2001 and 2002), only 34% of treated hypertensive patients were adequately controlled [22]. Two smaller regional studies showed that among treated hypertensive patients, 49.5 and 49.1% (of 344 and 103 treated patients, respectively) were controlled [23, 24]. In the Hypertension Study in General Practice in Hellas (HYPERTENSHELL; 11, 950 participants), which was conducted during 2002-2004 across Greece, 32.8% of treated hypertensive patients were adequately controlled [25]. Most of these levels of control are below those achieved in the current study (i.e. 49.2%).
The 2003 and 2007 European Society of Hypertension / European Society of Cardiology guidelines state that the management of hypertension should depend on the estimated global vascular risk [13, 26]. This is based on the fact that only 20% of hypertensive patients have hypertension alone while the majority has 1 or more additional risk factors [27, 28]. This coexistence of risk factors, which was also evident in our study, produces a total vascular risk that is greater than the sum of the individual factors [29, 30]. It is estimated that in patients with BP of 140-159/90-99 mmHg and at least 1 additional risk factor, reducing SBP by 12 mmHg for 10 years will prevent 1 death for every 11 treated hypertensive patients [31].
In our study, 59.7% of hypertensive patients had dyslipidemia at baseline. The increase in the use of statins (from 24.8% to 39.2%, p<0.0001) contributed to the significantly improved lipid profile (total cholesterol, LDL-C and TG but not HDL-C levels). The co-existence of hypertension and dyslipidemia confers a greater increase in vascular risk than would be expected with either risk factor alone [32]. Two trials evaluated the benefits associated with the use of statins specifically in hypertensive patients. In the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial - Lipid-Lowering Trial (ALLHAT-LLT) (10, 355 hypertensive patients), pravastatin (40 mg/d) did not reduce CHD events, stroke and all cause mortality significantly more than “usual care” [33]. In contrast, in the Anglo-Scandinavian Cardiac Outcomes Trial - Lipid Lowering Arm (ASCOT-LLA; 10, 305 hypertensive patients with at least 3 other vascular risk factors but free of CHD), atorvastatin (10 mg/d) significantly reduced vascular events (36%; p=0.0005) and stroke (27%; p=0.024) compared with placebo [34]. This discrepancy may be related to several factors, among which is the extent of LDL-C lowering, which was greater in the ASCOT-LLA trial (17 vs. 29% relative reduction) [33, 34]. A recent meta-analysis showed that statin therapy reduces vascular morbidity and mortality to the same extent in hypertensive and normotensive patients [35]. There is evidence in secondary prevention that the combination of a statin with angiotensin converting enzyme inhibitors reduces the risk of vascular events more than either drug alone [36]. Furthermore, statins may beneficially influence the BP and some antihypertensive drugs can have a beneficial, neutral or adverse effect on the lipid profile [37-39]. However, we cannot comment on these potential effects using our results.
Current guidelines state that low-dose ASA should be prescribed to hypertensive patients with established CVD, as well as to patients free of CVD but older than 50 years, with a moderate increase in serum creatinine levels or with a high vascular risk [13]. Antiplatelet therapy should be started after achievement of BP control [13]. The Hypertension Optimal Treatment (HOT) trial showed that treatment of hypertensive patients with ASA resulted in a 15% reduction in major vascular events and a 36% reduction in acute MI [40]. There was no effect on stroke and no increase in intracerebral haemorrhage but a 65% increased risk of major hemorrhagic events was observed [40]. The relatively low percentage of patients receiving ASA in our study (18.5%) may reflect physician reluctance to accept an increased risk of bleeding in a primary prevention setting.
In the UK Prospective Diabetes Study (UKPDS), hypertensive patients with T2DM benefited from intensive blood glucose control mainly in terms of microvascular complications (a 37% reduction in microvascular complications for each 1% reduction in HbA1c) [41]. Other studies showed that improved glycemic control (by lifestyle measures and pharmaceutical interventions) also protects against macrovascular complications [42, 43]. In our study, 16% of hypertensive patients had T2DM and pharmacologic treatment in combination with lifestyle measures resulted in a significant reduction in HbA1c (from 7.4% to 6.1%, p<0.0001). The prevalence of T2DM at the end of study decreased by 7.5% (from 16% to 14.8%, p=0.09).
In our study, 37.8% of men and 45.9% of women had MetS at baseline. The prevalence of MetS in the Western World, including Mediterranean Countries, is high [44] but awareness, treatment and control of MetS and its components remain poor [45-47]. MetS confers an increased risk for vascular morbidity and mortality [48-53] and all-cause mortality [48], even in Mediterranean Countries and in the absence of T2DM and/or clinically evident CVD [49, 50, 54, 55]. The combination of lifestyle interventions and drug treatment for all vascular risk factors in our patients resulted in a significant reduction in the prevalence of MetS (from 37.8% to 29.3% in men and from 45.9% to 34.2% in women; p<0.0001 for both comparisons).
LIMITATIONS
In this study there was no control group due to ethical restrictions, as explained above. Each patient acted as his/her own control and had been on a regular treatment regime for an average of 23 months (range: 8-72 months) prior to inclusion in the study. The estimated fall in vascular risk in hypertensive patients was calculated after intervention for 6 months. The observed benefits may decrease if adherence to lifestyle measures and pharmacological treatment deteriorates in the long run. The PROCAM and Framingham calculations were based on a treated population both at baseline and at the end of the study. Twenty seven patients did not attend the final visit (after 6 months). These patients represent only 3.9% of the baseline population and therefore they were not included in the final analysis. Nevertheless, this should be considered among the limitations of the study.
CONCLUSIONS
This is the first study to increase adherence to multiple interventions in hypertensive patients on an outpatient basis, both in primary care and teaching hospitals. Simple, relatively low cost measures (e.g. educating physicians and patients, distributing printed guidelines/brochures and completing a 1-page form) were associated with achieving treatment goals for multiple vascular risk factors. Further work is needed to establish the cost effectiveness and long-term benefits of such programs.
CO-INVESTIGATORS
We thank the following physicians for providing patient data. V. Chatzikaloudi and Ch. Kamilali, Health Centre (HC) of Sidirokastro; P. Psaris, Department of Internal Medicine, Genimatas Hospital, Thessaloniki; E. Papastefanou, Department of Internal Medicine, Serres Hospital; A. Taplidis, Department of Internal Medicine, Naousa Hospital; P. Volonakis, Nephrology Unit, Department of Internal Medicine, Agios Dimitrios Hospital, Thessaloniki; E. Thoma, HC of Deskati; K. Ioannou, HC of Sohos; E. Mandikou, HC of Langadas; K. Gaitanaki, HC of Krya Vrysi; A. El. Hatimi, HC of Zichni; E. Hartamba, HC of Sappes; K. Stefanidou, HC of Skydra; G. Pipertzis, HC of Strymoniko.
ACKNOWLEDGEMENTS
We thank the following employees of the Regional (Bureau) Authority of the Ministry of Health for Northern Greece-Central Macedonia for their contribution in the study. A. Trichopoulou, Department Head and D. Florinis, A. Kakafika.
DECLARATION OF INTEREST
This study was conducted independently under the auspices of the Regional Bureau (Authority) of the Ministry of Health for Northern Greece - Central Macedonia. No company or institution supported it financially. Some of the authors have given talks, attended conferences and participated in other trials or advisory boards sponsored by various pharmaceutical companies.


