All published articles of this journal are available on ScienceDirect.
Arterial Stiffness in Resistant Hypertension: From Physiology to Medical Practice
Abstract
Considering that the risks of cardiovascular disease still pose a significant challenge despite preventive and therapeutic efforts, we need new pathophysiological models for a better understanding of risks and treatment based on new concepts. Arterial Stiffness (AS) has been increasingly studied as an independent cardiovascular risk factor. The mechanisms by which AS develops are not yet fully understood. However, it clearly involves not only structural changes but also endothelial functional changes. There are several clinical studies showing that vascular damage is an important risk factor for structural and functional injury of high-flow organs. Carotid-femoral Pulse Wave Velocity (PWV-cf) has been considered the gold standard for the evaluation of AS, with a large body of evidence demonstrating its association with cardiovascular disease, regardless of traditional risk factors. Based on the impact of high PWV-cf on cardiovascular prognosis, achieving a decrease in PWV would possibly reduce cardiovascular events. However, the significance of lowering AS to reduce cardiovascular events under treatment remains to be unequivocally demonstrated. Regarding resistant hypertension, it shares risk factors with AS, including advanced age, endothelial dysfunction, left ventricular hypertrophy, obesity, diabetes, and chronic kidney disease. On the other hand, increased AS in resistant hypertensives presents a two-way interaction, where uncontrolled blood pressure results in progressive vascular damage while vascular stiffness increases blood pressure, making its control more difficult and establishing a vicious circle. In this scenario, the better the understanding of the complex interaction of factors to AS development, the more treatment options should become clinically available.
1. INTRODUCTION
Resistant Hypertension is defined as off-target blood pressure levels despite the use of three different classes of anti-hypertensive drugs in optimized and tolerable doses for more than 3 to 6 months. Ideally, these drugs should be a thiazide diuretic, a Renin-Angiotensin-Aldosterone (RAA) system inhibitor, and a long-acting calcium channel blocker. Individuals with 4 or more drugs are also included in this definition [1, 2]. Pathophysiology is multifactorial, but it is mainly characterized by hyperaldosteronism status with resulting volume overload. The effects of aldosterone are mediated by genomic and non-genomic mechanisms through interaction with mineralocorticoid receptors. In addition to its classic effect on the balance of electrolytes in the kidney, aldosterone also causes inflammation and ventricular remodeling, as well as vascular damage characterized by endothelial dysfunction and Arterial Stiffness (AS) [3]. On the other hand, high blood pressure generates mechanical and oxidative stress, production of collagen, and degradation of elastin in the arterial wall [4]. It then creates a vicious circle of inflammation and vascular damage [4].
This strong association between hypertension and AS justifies its investigation not only as a predictor of fatal and non-fatal cardiovascular events but also as a more preemptive approach, aiming for preventive measures of lifestyle changes and pharmacological treatments to increase vascular protection [4].
1.1. Resistant and Refractory Hypertension
It is estimated that the worldwide prevalence of resistant hypertension reaches 10-20% of hypertensive patients [1, 2]. Its pathophysiology is multifactorial and comprises hyperactivity of the sympathetic nervous system and RAA system, endothelial dysfunction, and AS, where aldosterone plays an important role in volume-dependent phenotype. It is also strongly related to resistance to anti-hypertensive therapy and associated with a higher incidence of cardiovascular events and hypertension-mediated organ damage, including increased AS [1, 2, 5].
Some bioactive markers are related to its hyperaldosteronism status, such as increased leptin and decreased adiponectin levels among obese patients, as well as increased TNF-alpha, playing a critical role in AS [6, 7].
On the other hand, refractory hypertension, which is considered an extreme phenotype of resistant hypertension, has sympathetic hyperactivity as its pathophysiological basis. By definition, these patients are already using a double diuretic blockade, including a mineralocorticoid receptor antagonist without blood pressure control [2, 8]. The scientific literature needs more studies assessing AS in this population.
1.2. Arterial Stiffness (AS)
More recent concepts suggest the existence of four pillars of cellular and molecular nature associated with AS: 1) stiffening of the extracellular matrix by increasing the collagen/elastin ratio and accumulation of proteoglycans, 2) phenotypic changes in vessel smooth muscle cells and stiffening, triggering transdifferentiation and activating focal adhesion dynamics and mechanotransduction, 3) inflammation and oxidative stress by increasing the expression of RAA system, cytokines, and chemokines, with resulting activation of macrophages and greater transdifferentiation of vascular smooth muscle cells, with increased expression of NADPH and oxidase activity, inducing mitochondrial dysfunction, 4) endothelial dysfunction with reduced availability of nitric oxide and modulation of molecular adhesion expression, causing altered contractility and infiltration and adhesion of circulating blood cells (Fig. 1) [9].
The arterial tree has two functions: conduction, taking blood from the left ventricle to the capillaries of organs and tissues, according to their needs, and damping, attenuating the pulsations generated by the heart so that capillary blood flow is continuous or almost continuous. The elastic conduction properties of arteries vary along the arterial tree, with proximal arteries being more elastic and distal arteries more rigid due to molecular, cellular, and histological heterogeneity. This heterogeneity means that the pressure wave propagating along this “viscoelastic tube” with numerous branches is progressively amplified due to wave reflection. Since distal arteries have a high level of resistance, the waves are reflected, and retrograde waves are generated, producing a secondary rise in diastolic blood pressure, increasing tissue perfusion, and protecting the periphery since less pulsatile energy is transmitted to the microcirculation. It particularly explains why an increase in AS increases central pulse pressure, with an associated increase in systolic blood pressure, a phenomenon observed in elderly individuals [9, 10].
Advanced age and hypertension accelerate arterial stiffening, reducing distensibility of the great arteries, resulting in dilation and stiffening. This is reflected in widened pulse pressure with left ventricular systolic overload and lower myocardial perfusion during diastole, increasing the risk of coronary events [9, 10].
1.3. Measuring the Degree of AS
The two most widely used methods described for clinical use and epidemiological studies are carotid-femoral Pulse Wave Velocity (PWV-cf) and pulse wave analysis [11], with PWV-cf measurement being considered the gold standard for assessing hypertension-mediated organ damage when measuring AS [5]. It is the most validated method due to its simplicity, accuracy, reproducibility and because it is a strong prognostic predictor [12]. Pulse wave velocity is inversely proportional to vessel compliance. The measurement is made using a semi-automatic device that uses mechanotransducers applied to the skin (applanation tonometry) at the site of palpation of the carotid and femoral arteries using the foot-to-foot method of the various waveforms. The cut-off value is > 10 m/s [13].
Nowadays, there is currently validated equipment for measuring central blood pressure and pulse wave velocity using the triple trigger method. Three brachial blood pressure measurements are taken at 3-minute intervals, and an algorithm is used in proprietary software to calculate central parameters, including central blood pressure, pulse wave velocity, augmentation index, cardiac output, and peripheral vascular resistance [14].
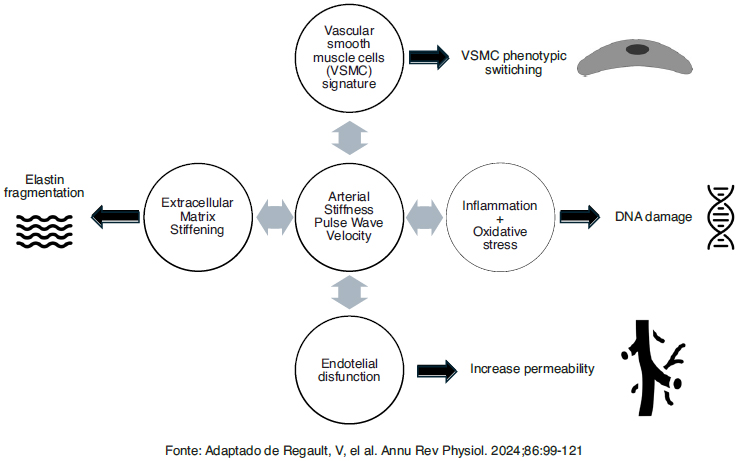
Presents a summary of these four pillars associated with arterial stiffness [9].
Available online under the terms of the Creative Commons Attribution Non-Commercial License 4.0.
1.4. AS and Resistant Hypertension
The stiffness of the great arteries is a natural consequence of the aging process. In addition to age, a set of clinical conditions are common to AS and resistant hypertension, including Black ethnicity, female sex at birth, obesity, diabetes, chronic kidney disease, and left ventricular hypertrophy [1, 2].
There are few studies directly assessing resistant hypertensive patients and AS, mostly in small populations, which are summarized in Table 1.
Martins et al. evaluated two groups of 90 resistant hypertensive patients with both controlled and uncontrolled blood pressure. They found that older age, higher aldosterone levels, higher AS, and more severe left ventricular hypertrophy characterize patients with uncontrolled resistant hypertension when compared to those with controlled resistant hypertension. The data of this study supports the hypothesis that the progression from the early stages of hypertension to severe and hard-to-control hypertension is characterized by progressive AS and left ventricular hypertrophy [15].
An observational study enrolled 54 participants: 17 with resistant hypertension (median age 60.5±12.4 years), 18 hypertensive (57.7±12.2 years), and 19 normotensive (54.7±11.9 years). Pulse wave velocity analysis was performed to measure central blood pressure, aortic augmentation pressure, mean blood pressure, and augmentation index (AIx). Central and office blood pressure were elevated in the group with resistant hypertension when compared with the other groups (p<0.05). All parameters analyzed were significantly increased in resistant hypertensive patients in comparison with other groups (p<0.001) [16].
A cross-sectional study comparing AS and central blood pressure in 141 patients with controlled resistant hypertension and controlled hypertensive patients without resistant hypertension did not find differences between the groups, therefore inferring that the increased cardiovascular risk of controlled resistant hypertensives should not be attributed to these core parameters [17].
Conversely, Figueiredo et al. [18] demonstrated a significant increase in PWV-cf in patients with resistant hypertension (n=44) when compared with controlled hypertensives (n=35) and normotensives (n=25). Since endothelial function can contribute to regulating the elasticity of the great arteries, the authors observed that a greater decrease in flow-mediated vasodilation of the brachial artery was more evident in patients with resistant hypertension compared to well-controlled hypertensive patients, inferring that micro and macrocirculation would be compromised in resistant hypertensives.
| Source\Refs | Population (N) | Intervention | Comparison | Stated Outcome/Findings |
|---|---|---|---|---|
| Barbaro NR et al. [24] | 51 | Measurement of plasma levels of TNF-α and arterial stiffness (PWV); treatment of HUVECs with TNF-α inhibitor (infliximab). | The effect of inhibition on HUVECs with serum from RHTN vs. normotensive subjects; HUVECs from normotensive and hypertensive individuals treated with infliximab. | Higher TNF-α levels and PWV in RHTN; positive correlation between TNF- α levels and PWV; increased apoptosis and ROS in HUVECs with RHTN serum; infliximab attenuated apoptosis but not ROS production. |
| Barbaro NR et al. [7] | 72 | Measurement of office BP, arterial stiffness (PWV), and plasma levels of inflammatory biomarkers (IL-6, IL-10, IL-1β, TNF-α, hs-CRP). | Comparison among RHTN, HTN, and NT patients. | Increased PWV in RHTN compared to HTN and NT; higher TNF-α levels in RHTN and HTN than in NT; higher frequency of increased IL-10 and IL-1β levels in RHTN compared to HTN and NT; IL-1β independently associated with PWV. |
| Cai A et al. [17] | 141 | Evaluation using PWA and cf-PWV. | Comparison between patients with controlled RHTN (using 4 or more antihypertensive medications) and controlled non-RHTN (using 3 or fewer antihypertensive medications). | No significant difference in central BP, AS, AP, augmentation index normalized for HR of 75 beats/minute (AIx@75), or cf-PWV between controlled RHTN and controlled non-RHTN groups. Controlled RHTN patients were more likely to be African American and had a higher prevalence of diabetes mellitus and congestive heart failure. The mean number of antihypertensive medications was greater in the controlled RHTN group. |
| Chedier B et al. [26] | 1048 | Characterization of the clinical profile and prevalence of RfHT before and after the introduction of spironolactone. | Comparison between RHTN and RfHT patients; comparison before and after the introduction of spironolactone. | Prevalence of RfHT increased from 13.9% to 17.6% after the introduction of spironolactone. RfHT patients were younger, more obese, and had lower AS and PAD after spironolactone use. Despite lack of BP control, spironolactone use was associated with a lower cardiovascular risk in RfHT patients |
| de Faria AP et al. [6] | 96 | APN and aldosterone levels, office and ABPM, flow-mediated dilation FMD, LVMI, and PWV. | UCRHTN and CRHTN. | UCRHTN subgroup had higher aldosterone levels, LVMI, and PWV; lower APN levels; and impaired FMD response. Brachial and ABPM pulse pressures were inversely associated with APN levels in UCRHTN patients. Aldosterone levels and PWV were also inversely associated with APN levels in UCRHTN patients. PWV was significantly influenced by APN level in UCRHTN subgroup in multivariate analysis. |
| de Faria AP et al. [23] | 149 | Measurement of plasma 8-isoprostane levels, FMD, and PWV. | Comparison between RHTN and well-controlled HT patients. | Significant inverse correlation between FMD and 8-isoprostane in RHTN. 8-isoprostane was a significant predictor of endothelial dysfunction (FMD ≤ median) in RHTN group. |
| Dudenbostel T et al. [25] | 44 | Measurement of 24-hour urinary normetanephrine levels, clinic and ambulatory HR, HR variability, arterial stiffness PWV, and SVR. | Comparison between patients with RfHT and those with controlled RHT. | RfHT patients were younger, more likely women, had higher urinary normetanephrine levels, higher clinic HR, higher 24-hour ambulatory HR, higher PWV, reduced HR variability, and higher SVR. These results suggest heightened sympathetic tone as a major contributor to antihypertensive treatment failure, indicating the need for effective sympatholytic therapies. |
| Figueiredo VN et al. [18] | 193 | Measurement of FMD using high-resolution ultrasound and PWV calculated from pulse transit time and distance between carotid and femoral arteries. | Comparison between RHTN, well-controlled HTN, and NT groups. | Significant differences in BP-adjusted PWV between RHTN and well-controlled. Strong correlation between FMD and PWV in well-controlled HTN and NT subjects, with a lower correlation in RHTN patients. |
| Lopes S et al. [29] | 57 | Evaluation of arterial stiffness using cf-PWV. Objective assessment of daily physical activity using accelerometers over 7 consecutive days. | Assessment of the association between daily physical activity levels and arterial stiffness in patients with RHTN. | Inverse correlations observed between cf-PWV and light-intensity physical and total daily physical. The correlation between light physical activity and cf-PWV remained significant after adjustment for systolic and diastolic BP but not after further adjustment for age. Higher levels of light-intensity and total physical activity were associated with lower arterial stiffness. However, these associations weakened or disappeared after adjustments for BP and age. |
| Lotufo PA et al. [19] | 4116 | Definition and identification of RHTN | Comparison between participants with RHTN and those without, focusing on demographic and clinical characteristics. | Participants with resistant hypertension were more likely to be older, black, less educated, poorer, and obese. Adjusted prevalence ratios showed associations with diabetes, reduced glomerular filtration rate, elevated albumin-to-creatinine ratio, increased cf-PWV, thicker common carotid intima-media, LVH, and atrial fibrillation. |
| Moreno B et al. [27] | 220 | Measurement of intima-media thickness and FMD using high-resolution ultrasound, LVMI by echocardiography, and arterial stiffness by cf-PWV. | Comparison between controlled vs. uncontrolled diabetes mellitus regarding vascular parameters and diabetes control. | Patients with uncontrolled diabetes mellitus had worse endothelial dysfunction and arterial stiffness. HbA1c levels independently predicted flow-mediated dilation and PWV in all patients with RHTN |
| Ritter AM et al. [3] | 303 | Investigation of genetic polymorphisms (I180V - rs5522 and MRc.-2G_C - rs2070951) in the NR3C2 gene, associated with mineralocorticoid receptor function variability. | Comparison of clinical and laboratory characteristics between AA and G carriers for rs5522, and AC, GG, and CG carriers for rs2070951. | Found increased levels of aldosterone and ambulatory BP in G carriers of rs5522 in RHTN patients. G carriers of rs5522 also showed higher prevalence of LVH. |
| Roderjan CN et al. [21] | 442 | Evaluation of serial changes in cf-PWV over a mean follow-up of 4.6 years. | Correlation of changes in clinic and ambulatory BP with changes in cf-PWV. | Median increase of 0.11 m/s per year in cf-PWV. Identified that changes in clinic SBP and 24-hour were significant correlates of cf-PWV changes. The white-coat effect also influenced cf-PWV changes. Older age, diabetes, higher waist circumference, and worse renal function were additional independent correlates of AS progression. |
| Roderjan CN et al. [28] | 376 | Assessment of AS measured by PWV and its association with OSA severity | Bivariate analysis comparing patients with and without moderate/severe OSA. | PWV values showed a progressive increase with the severity of apnea. Significant differences in PWV were observed between patients without/mild apnea and those with moderate/severe apnea, particularly among true RHTN patients, women, and those with uncontrolled nocturnal systolic BP. |
| Schnaubelt S et al. [10] | 54 | Measurement of aortic AIx | Alevels between RHTN, CH, and NC groups | Aortic pulse pressure, MAP, and aortic augmentation pressure were significantly elevated in RH patients. AIx was markedly increased in RH compared to CH and NC |
A large epidemiological study revealed associations of resistant hypertension with PWV-cf, the intima-media thickness of the common carotid artery, and neck circumference, which are related to Obstructive Sleep Apnea (OSA), a potential cause of resistant hypertension. AS was related to resistant hypertension regardless of covariates such as age, sex at birth, ethnicity, body mass index, and neck circumference [19].
Another cross-sectional study evaluating AS in 600 patients of a large cohort of resistant hypertensive patients found a prevalence of increased PWV-cf of 28% in this population. Older age, diabetes, moderately increased albuminuria, low HDL-cholesterol, and lower nocturnal dipping are independently associated with an increase in AS [20]. A longitudinal study in this same cohort, with a mean follow-up of 4.6 years in 224 patients, provided evidence that changes in both office and ambulatory blood pressure contribute to the progression or regression of aortic stiffness in resistant hypertension, although the exacerbated white coat effect, usually present in this phenotype, seems to have at least partially obscured the beneficial effect of lowering blood pressure. Older age, diabetes, abdominal obesity, and worse renal function are also independently associated with the progression of AS in resistant hypertension [21].
In a review, Alsharari et al. [22] highlighted the role of inflammatory markers increasing AS in resistant hypertension, such as increased oxidative stress [23], as well as TNF-alpha [24] and increased cytokines [7].
1.5. AS and Refractory Hypertension
Only two studies have assessed AS in refractory hypertension [25, 26], with controversial results. Dudenbostel [25] evaluated 15 refractory hypertensive patients versus 29 patients with controlled resistant hypertension, finding higher sympathetic activity as a cause of refractoriness (higher levels of urinary metanephrines and higher clinical and ambulatory heart frequency), as well as higher pulse wave velocity and systemic vascular resistance. In turn, Chedier [26] evaluated 688 resistant and 147 refractory hypertensive patients and found a higher prevalence of increased pulse wave velocity among resistant ones (21.4% vs. 13.3%, p<0.05) despite uncontrolled blood pressure. This finding was attributed to the mandatory use of spironolactone by refractory patients.
2. DISCUSSION
From the limited data available, we can observe a tendency for AS to actually be higher than expected for age and blood pressure level in people with resistant hypertension, but there are some confounding factors for this analysis. On the one hand, the higher prevalence of comorbidities such as diabetes mellitus, chronic kidney disease, and sleep apnea worsen vascular damage and, on the other hand, physical activity and use of spironolactone can induce reverse vascular remodeling, thus reducing arterial stiffness [22, 26-30].
Uncontrolled blood pressure is one of the factors responsible for AS, which in turn worsens endothelial dysfunction and vascular remodeling, establishing a vicious circle that makes blood pressure control harder [22]. Renin-angiotensin-aldosterone system hyperactivity directly contributes to the increase in AS, with angiotensin II leading to smooth muscle cell hypertrophy and collagen accumulation, while secondary hyperaldosteronism activates the growth of the extracellular matrix by fibroblasts [22].
Possibly, interventions used in the treatment of resistant hypertension may act directly to block the four pillars that lead to arterial stiffness (Fig. 1). In addition to the usual non-pharmacological interventions, such as physical activity, which is known to reduce inflammation and oxidative stress [29], there is a high prevalence of Obstructive Sleep Apnea among patients with resistant [31] and refractory hypertensives [32]. Consequently, many of them use Continuous Positive Airway Pressure (CPAP), which is also capable of inhibiting the RAA system, decreasing sympathetic activity, and reducing oxidative stress. Apparently, CPAP is not capable of reducing arterial stiffness measured by PWV, but it appears to be capable of preventing its progression [33].
Regarding pharmacological treatment, by definition, resistant hypertensive patients should be using inhibitors of the RAA system [1, 2], in addition to the use of spironolactone, well established as the fourth drug, used to control blood volume and reduce hyperaldosteronism status. Both drugs reduce inflammation and endothelial dysfunction [34].
CONCLUSION
Patients with chronic conditions often have multiple comorbidities, including other cardiovascular diseases, thus facing numerous challenges in managing their conditions. The association between AS and uncontrolled blood pressure makes the control of blood pressure levels even more difficult and challenging in this population, and consequent greater morbidity and mortality from cardiovascular or other causes.
The progression of AS shares several clinical characteristics and physiological pathways with resistant or refractory hypertension. In addition to age or sex as non-modifiable factors, hyperactivity of renin-angiotensin-aldosterone and sympathetic systems as modifiable paths seem to be one of the main starting points in the treatment of these conditions.
Thus, we believe that the measurement of Carotid-Femoral Pulse Wave Velocity should be included in the routine diagnosis and follow-up of resistant and refractory hypertension.
FUTURE DIRECTIONS
Robust, multicenter prospective longitudinal studies involving resistant and refractory hypertensive patients are needed to evaluate AS, its evolution in this population, white-coat effect relationship, and true prognostic impact. This could help guide new therapeutic modalities and even predict results after renal denervation, and making it possible to prevent outcomes such as all-cause mortality, cardiovascular and renal events.
AUTHORS’ CONTRIBUTION
It is hereby acknowledged that all authors have accepted responsibility for the manuscript's content and consented to its submission. They have meticulously reviewed all results and unanimously approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| AS | = Arterial Stiffness |
| PWV-cf | = Pulse Wave Velocity |
| RAA | = Renin-Angiotensin-Aldosterone |
ACKNOWLEDGEMENTS
Declared none.


