All published articles of this journal are available on ScienceDirect.
Long-Term Outcomes of Patients Undergoing Implantation of Long and Short Second-Generation Drug-Eluting Stents
Abstract
Introduction
Despite the introduction of first-generation drug-eluting stents, lesion length has remained a predictor of target lesion revascularization. To address this limitation, stents with thinner struts, more advanced polymers, and drugs with more controlled release were developed. However, the impact of second-generation drug-eluting stent (DES2) length on clinical outcomes remains uncertain.
Objective
The objective of this study was to evaluate the impact of DES2 length on long-term safety and efficacy by comparing outcomes between patients who underwent implantation of long and short DES2.
Methods
This observational, retrospective, non-randomized, single-center study included all patients who received only DES2 from January 1, 2011, to December 31, 2017. Patients were divided into two groups based on the length of the implanted DES2: 1) long stent group (LSG) with at least one stent measuring 30 mm or longer, and 2) short stent group (SSG) with one or more stents measuring 20 mm or shorter without overlap. The primary endpoint was target lesion failure (TLF) during a three-year follow-up period.
Results
A total of 278 patients were assigned to the SSG (mean stent length: 19.32±8.6 mm), while 436 patients were assigned to the LSG (mean stent length: 55.38±23.3 mm). The LSG showed a significantly higher incidence of TLF: 16.7% versus 10.4% [SHR (95%CI): 1.78 (1.15-2.76), p=0.01]. No significant differences were observed in the secondary endpoints.
Conclusion
Despite technological advances, the use of DES2 measuring 30 mm or longer has been associated with a higher TLF rate. Therefore, the implantation of longer DES2, compared with DES2 measuring 20 mm or shorter, significantly impacted long-term clinical outcomes related to TLF, while secondary outcomes were not affected.
1. INTRODUCTION
According to the Global Burden of Disease 2021 Study, cardiovascular disease was the leading cause of death worldwide. Among these, ischemic heart disease ranks as the primary cause, with an age-standardized death rate of 108.7 deaths per 100,000 population. In addition, ischemic heart disease was the second leading cause of disease burden, accounting for 188.3 million disability-adjusted life-years (DALYs) [1]. Regional data indicate a global trend toward increasing percutaneous coronary intervention (PCI) volumes and decreasing coronary artery bypass grafting volumes, driven by advances in PCI technology and changes in clinical practice [2, 3].
Approximately 20% of all patients with obstructive coronary artery disease have long lesions, defined as those measuring 25 mm or more [4]. Complete lesion coverage, achieved using either longer stents or overlapping shorter stents, is the current PCI strategy to reduce restenosis rates [5].
Even with the introduction of first-generation drug-eluting stents, atherosclerotic lesion length has remained a predictor of restenosis, stent thrombosis, and, consequently, target lesion revascularization (TLR) [6].
To address these limitations, second-generation drug-eluting stents (DES2) were developed, incorporating thinner struts, more advanced polymers (biocompatible or bioabsorbable), and drugs with more controlled release [7]. Compared with first-generation drug-eluting stents, DES2 has significantly reduced stent thrombosis and TLR rates [8].
However, the impact of DES2 length on clinical outcomes remains uncertain, and previous studies have reported inconsistent results [9-17]. Hence, this study aimed to evaluate the impact of DES2 length on long-term safety and efficacy by comparing outcomes between patients who underwent implantation of long and short DES2.
2. MATERIALS AND METHODS
2.1. Study Population
This observational, retrospective, non-randomized, single-center study included all patients who received only DES2 from January 1, 2011, to December 31, 2017, at a single center in Blumenau, Brazil.
Patients were divided into two groups based on the length of the implanted DES2: 1) long stent group (LSG), which included patients with at least one stent measuring 30 mm or longer, and 2) short stent group (SSG), which included patients with one or more stents measuring 20 mm or shorter without overlap.
Patients with unsuccessful procedures, vein graft interventions, or loss to follow-up were excluded. No additional exclusion criteria were applied to reflect a real-world scenario.
Demographic and clinical characteristics are summarized in Table 1. The ages of the patients ranged from 31 to 98 years. No statistically significant differences were observed between the two groups regarding dual antiplatelet therapy (DAPT) or its duration.
| Short Stents | Long Stents | p | |||
|---|---|---|---|---|---|
| Number | % | Number | % | ||
| Mean age (Years) ± Sd | 63.1 ± 11 | 62.9 ± 11.6 | 0.824 | ||
| Gender | |||||
| Female | 108 | 38.8 | 139 | 31.9 | |
| Male | 170 | 61.2 | 297 | 68.1 | 0.063 |
| Clinical Presentation | |||||
| Chronic coronary disease | 128 | 46 | 153 | 35.1 | |
| Non-ST-elevation ACS | 108 | 38.8 | 198 | 45.4 | |
| ST-elevation myocardial infarction | 32 | 11.5 | 69 | 15.8 | |
| APE/CS/SRCA | 10 | 3.6 | 16 | 3.7 | 0.028 |
| Risk Factors | |||||
| Active smoking | 40 | 14.4 | 84 | 19.3 | 0.105 |
| Diabetes mellitus | 115 | 41.4 | 165 | 37.8 | 0.387 |
| Dyslipidemia | 190 | 68.3 | 250 | 57.3 | 0.003 |
| Hypertension | 216 | 77.7 | 328 | 75.2 | 0.472 |
| Clinical Background | |||||
| Previous myocardial infarction | 40 | 14.4 | 76 | 17.4 | 0.3 |
| Previous PCI | 50 | 18 | 104 | 23.9 | 0.076 |
| Previous CABG | 35 | 12.6 | 42 | 9.6 | 0.218 |
| Comorbidities | |||||
| Atrial fibrillation | 14 | 5 | 16 | 3.7 | 0.445 |
| Cancer | 7 | 2.5 | 13 | 3 | 0.819 |
| Chronic obstructive pulmonary disease | 9 | 3.2 | 16 | 3.7 | 0.837 |
| Dialytic chronic kidney disease | 2 | 0.7 | 10 | 2.3 | 0.141 |
| Non-dialytic chronic kidney disease* | 11 | 4 | 16 | 3.7 | 0.843 |
| Heart failure | 4 | 1.4 | 7 | 1.6 | 1 |
| Peripheral arterial disease | 18 | 6.5 | 29 | 6.7 | 1 |
| Stroke | 11 | 4 | 20 | 4.6 | 0.851 |
2.2. Coronary Interventions and Antiplatelet Therapy
All procedures were performed according to current standard techniques.
All patients received loading doses of DAPT consisting of acetylsalicylic acid combined with either clopidogrel, ticagrelor, or prasugrel as early as possible. For patients already on DAPT, standard maintenance doses were continued. DAPT was recommended for twelve months in cases of acute coronary syndrome and for six months in cases of elective interventions. After these specified periods, acetylsalicylic acid maintenance therapy was advised. In cases of documented allergy to acetylsalicylic acid, indefinite maintenance with a P2Y12 receptor antagonist was recommended.
During procedures, intravenous unfractionated heparin was administered at a dose of 100 IU/kg, and abciximab was used at the operator’s discretion.
2.3. Definitions
Angiographic success was defined according to the guidelines for PCI established by the Society for Cardiovascular Angiography and Interventions [18].
Myocardial infarction was defined according to the criteria set forth by the Fourth Universal Definition [19].
Bleeding was classified according to the Bleeding Academic Research Consortium (BARC) definitions [20].
Major adverse cardiovascular events (MACE) were defined as a composite of all-cause death, any myocardial infarction, and TLR.
As established by the Academic Research Consortium-2, target lesion failure (TLF) was defined as a composite of cardiovascular death (CVD), target vessel myocardial infarction, and TLR. The definitions of all other endpoints followed the guidelines outlined in this consensus document [21].
2.4. Objectives
The objective of the study was to evaluate whether the length of DES2 affects long-term safety and efficacy by comparing the outcomes of patients who underwent implantation of long and short DES2.
The primary endpoint was TLF during a three-year follow-up period. Secondary endpoints included: 1) safety outcomes: all-cause death, CVD, any myocardial infarction, definite stent thrombosis, BARC-3 and -5 bleeding, and stroke; 2) efficacy outcomes: TLR, target vessel revascularization (TVR), and any coronary revascularization; and, 3) MACE during a three-year follow-up period.
2.5. Clinical Follow-up
Long-term clinical follow-up was conducted by reviewing a pre-existing database. Demographic, clinical, and procedural data were collected by the treating physician after PCI.
Regular telephone follow-up contacts were made by the study team for all patients who underwent PCI. The first two contacts were made at six-month intervals, followed by annual contacts. The last recorded contact was considered the end of the follow-up period.
2.6. Statistical Analysis
Data were analyzed using Stata/SE version 14.1 statistical software. Quantitative variables were reported as means with standard deviations, whereas categorical variables were reported as percentages. Quantitative variables were compared between groups using Student's t-test for independent samples or a non-parametric Mann-Whitney test. Categorical variables were compared using Fisher's exact test or chi-squared test.
Univariate and multivariate analyses of variables associated with the incidence of TLF were adjusted using Fine and Gray models. Variables with a p < 0.05 in the univariate analysis were included in the multivariate analysis. This model was also used to compare the groups with respect to the incidence of CVD, any myocardial infarction, definite stent thrombosis, BARC-3 and -5 bleeding, stroke, TLR, TVR, and any coronary revascularization. The estimated measure of association was the subdistribution hazard ratio (SHR).
Cox regression models were adjusted for the analysis of variables associated with all-cause death and time until the occurrence of MACE. The estimated measure of association was the hazard ratio (HR).
For all measures of association, 95% confidence intervals (95%CI) were presented, and the significance of variables was assessed using the Wald test. Kaplan-Meier and cumulative incidence curves were used to describe the results. A value of p < 0.05 was considered statistically significant.
3. RESULTS
During the predefined seven-year period, 801 individuals underwent PCI with DES2 of the lengths relevant to this study. Eighty-seven patients (10.9%) were excluded due to loss to follow-up. Among those who completed follow-up, 278 patients (38.9%) were assigned to the SSG, while 436 patients (61.1%) were assigned to the LSG.
The mean follow-up duration was 3.16 years for the SSG and 2.95 years for the LSG (p = 0.288).
Angiographic and PCI characteristics are summarized in Table 2. Radial access increased only in the final period of the study. Stent overlap, which was only allowed in the LSG, was required in 23.4% of patients. The main results of the study are presented in Figs. (1-8). The LSG showed a significantly higher incidence of TLF compared with the SSG: 16.7% versus 10.4% [SHR (95%CI): 1.78 (1.15 - 2.76), p = 0.01].
Further, no significant differences were observed in the secondary endpoints (for LSG and SSG, respectively).
(1) Safety outcomes were as follows:
• All-cause death: 16.3% versus 13.3% [HR (95%CI): 1.27 (0.85 - 1.89), p = 0.238],
• CVD: 10.6% versus 7.2% [SHR (95%CI): 1.51 (0.89 - 2.55), p = 0.126]
• Any myocardial infarction: 5% versus 4.7% [SHR (95%CI): 1.14 (0.56 - 2.29), p = 0.721],
• Definite stent thrombosis: 1.6% versus 1.1% [SHR (95%CI): 1.6 (0.4 - 6.43), p = 0.51],
• BARC-3 and -5 bleeding: 2.1% versus 1.1% [SHR (95%CI): 1.9 (0.52 - 7.02), p = 0.335],
• Stroke: 0.7% versus 1.8% [SHR (95%CI): 0.38 (0.09 - 1.58), p = 0.182]
(2) Efficacy outcomes were as follows:
• TLR: 3.4% versus 1.4% [SHR (95%CI): 2.49 (0.82 - 7.54), p = 0.106],
• TVR: 3.9% versus 2.2% [SHR (95%CI): 1.88 (0.74 - 4.76), p = 0.185],
• Any coronary revascularization: 7.8% versus 4.3% [SHR (95%CI): 1.9 (0.98 - 3.67), p = 0.057],
(3) MACE: 24.8% versus 19.4% [HR (95%CI): 1.38 (0.99 - 1.92), p = 0.054].
| Short Stents | Long Stents | p | |||
|---|---|---|---|---|---|
| Number | % | Number | % | ||
| Treated Artery | 314 | 634 | |||
| Left anterior descending | 149 | 47.5 | 273 | 43.1 | |
| Right coronary | 73 | 23.2 | 185 | 29.2 | |
| Circumflex | 68 | 21.7 | 138 | 21.8 | |
| Left main trunk | 21 | 6.7 | 28 | 4.4 | |
| Internal mammary artery graft | 2 | 0.6 | 1 | 0.2 | |
| Intermediate | 1 | 0.3 | 9 | 1.4 | 0.085 |
| Number of Vessels | |||||
| Single-vessel patients | 244 | 87.8 | 266 | 61 | |
| Multivessel patients | 34 | 12.2 | 170 | 39 | <0.001 |
| Number of Lesions | 351 | 857 | |||
| Patients with one lesion | 223 | 80.2 | 174 | 39.9 | |
| Patients with more than one lesion | 55 | 19.8 | 262 | 60.1 | <0.001 |
| Treated lesions/patient ± SD | 1.24 ± 0.54 | 1.96 ± 1.05 | <0.001 | ||
| Lesions Characteristics | |||||
| Bifurcation | 64 | 23 | 100 | 22.9 | 1 |
| Chronic total occlusion | 0 | 0 | 15 | 3.4 | <0.001 |
| In-stent restenosis | 20 | 7.2 | 56 | 12.8 | 0.018 |
| Ostial | 45 | 16.2 | 76 | 17.4 | 0.684 |
| Severe calcification | 15 | 5.4 | 39 | 8.9 | 0.084 |
| Severe tortuosity | 5 | 1.8 | 8 | 1.8 | 1 |
| Thrombus | 10 | 3.6 | 30 | 6.9 | 0.068 |
| Left Ventricle | |||||
| Normal or discreet dysfunction | 224 | 84.8 | 306 | 71.8 | |
| Moderate or severe dysfunction | 40 | 15.2 | 120 | 28.2 | <0.001 |
| Devices | |||||
| Fractional flow reserve | 3 | 1.1 | 2 | 0.5 | 0.383 |
| Thrombus aspiration catheter | 10 | 3.6 | 17 | 3.9 | 1 |
| Pre-dilatation | 202 | 72.7 | 368 | 84.4 | <0.001 |
| Post-dilatation | 86 | 30.9 | 270 | 61.9 | <0.001 |
| Kissing balloon | 36 | 12.9 | 64 | 14.7 | 0.581 |
| Stents | 353 | 838 | |||
| Patients with one stent | 214 | 77 | 177 | 40.6 | |
| Patients with more than one stent | 64 | 23 | 259 | 59.4 | <0.001 |
| Stents/patient ± SD | 1.27 ± 0.55 | 1.92 ± 1.01 | <0.001 | ||
| Mean diameter (mm) ± SD | 2.95 ± 0.54 | 2.94 ± 0.42 | 0.943 | ||
| Mean total length (mm)/patient ± SD | 19.32 ± 8.6 | 55.38 ± 23.3 | <0.001 | ||
| Strut Thickness | |||||
| ≤70 µm | 95 | 26.9 | 287 | 34.2 | |
| >70 µm | 258 | 73.1 | 551 | 65.8 | 0.014 |
| Polymer | |||||
| Bioabsorbable | 148 | 41.9 | 323 | 38.5 | |
| Durable | 205 | 58.1 | 515 | 61.5 | 0.276 |
| Drugs | |||||
| Biolimus | 30 | 8.5 | 26 | 3.1 | |
| Everolimus | 140 | 39.7 | 350 | 41.8 | |
| Sirolimus | 118 | 33.4 | 297 | 35.4 | |
| Zotarolimus | 65 | 18.4 | 165 | 19.7 | 0.001 |
| Results | |||||
| Complete revascularization | 219 | 78.8 | 316 | 72.5 | 0.063 |
| Discrete residual lesions | 40 | 14.4 | 105 | 24.1 | 0.002 |
| Reversed no-reflow/slow flow | 2 | 0.7 | 7 | 1.6 | 0.494 |
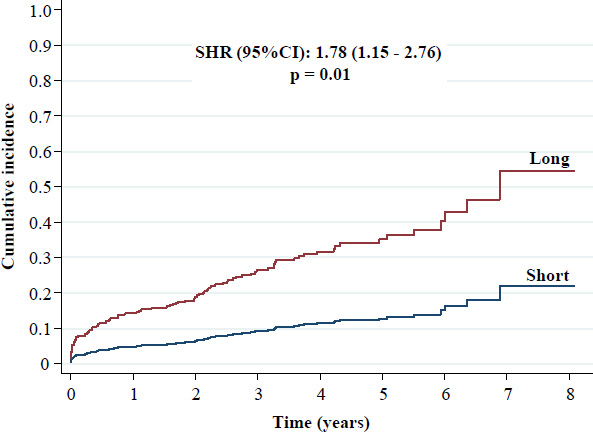
Cumulative incidence of target lesion failure.
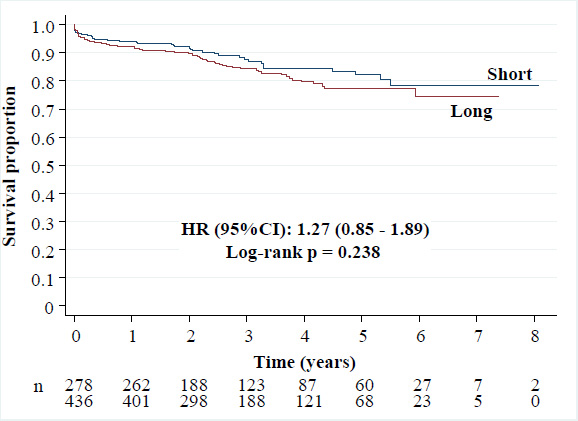
Kaplan-Meier curves of all-cause death.
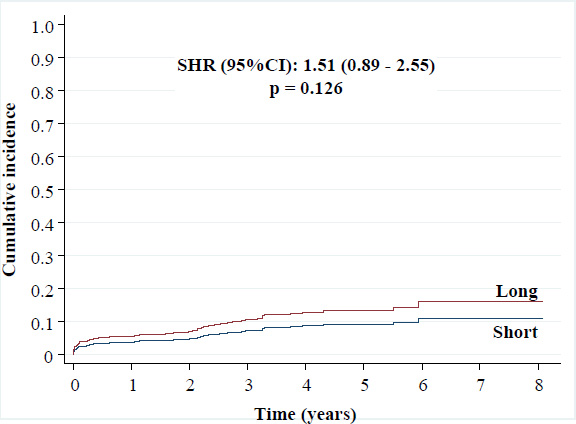
Cumulative incidence of cardiovascular death.
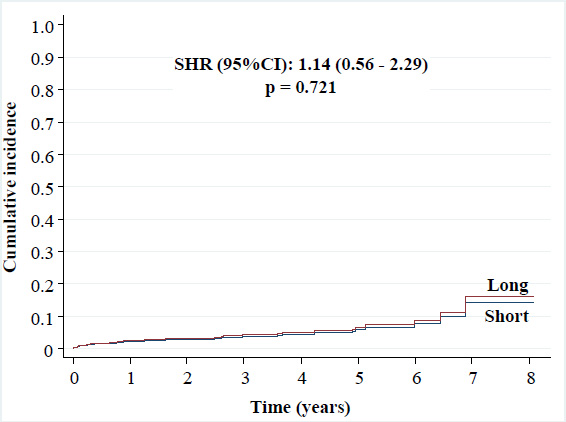
Cumulative incidence of any myocardial infarction.
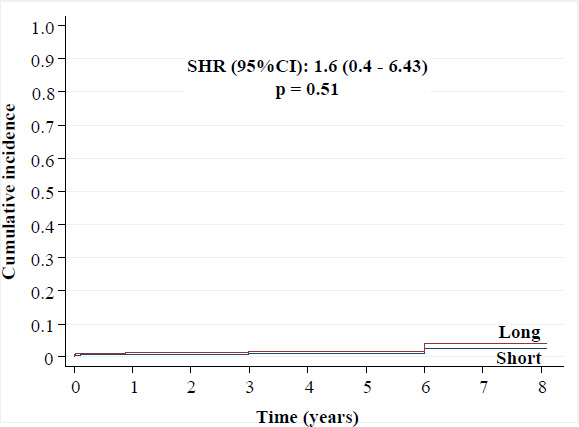
Cumulative incidence of definite stent thrombosis.
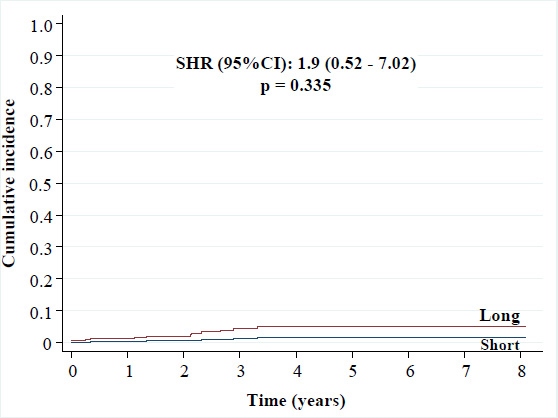
Cumulative incidence of BARC-3 and -5 bleeding.
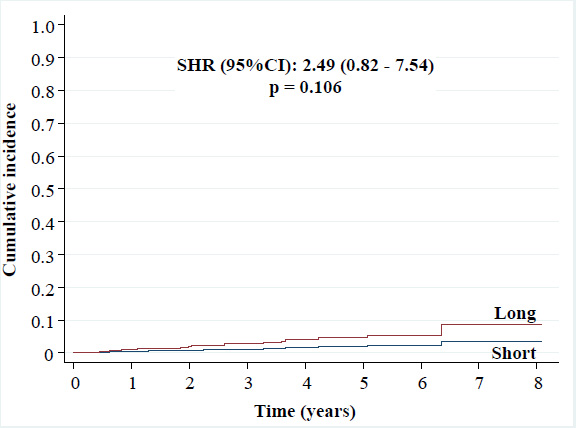
Cumulative incidence of target lesion revascularization.
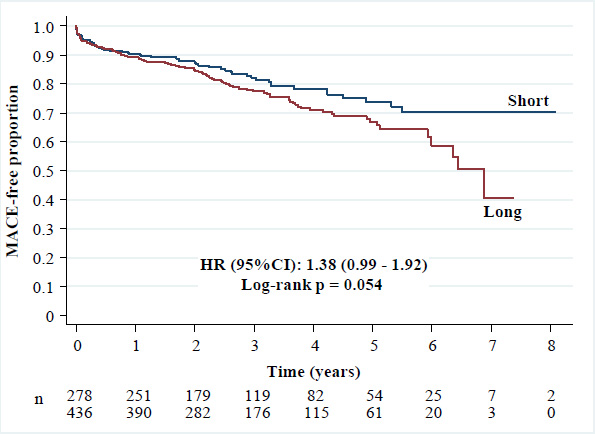
Kaplan-Meier curves of major adverse cardiovascular events.
Excluding patients with severe clinical conditions (acute pulmonary edema, cardiogenic shock, or successfully resuscitated cardiac arrest), the LSG still showed a higher TLF rate as follows: 14.8% versus 9.3% [SHR (95%CI): 1.7 (1.07 - 2.73), p = 0.026]. These sixteen individuals (3.7%) from the LSG and ten (3.6%) from the SSG had a mortality rate of 80.8% during the entire follow-up period.
Multivariate analysis confirmed that complete revascularization was a protective factor for TLF [SHR (95%CI): 0.54 (0.34 - 0.86), p = 0.009]. Additionally, the following independent predictors of TLF were identified: the LSG [SHR (95%CI): 1.8 (1.06 - 3.06), p = 0.029], restenosis treatment [SHR (95%CI): 2.06 (1.19 - 3.55), p = 0.01], previous heart failure [SHR (95%CI): 2.79 (1.32 - 5.89), p = 0.007], and severe clinical conditions (acute pulmonary edema, cardiogenic shock, or successfully resuscitated cardiac arrest) [SHR (95%CI): 8.46 (3.76 - 19.0), p < 0.001].
4. DISCUSSION
The major findings of this study were as follows: 1) DES2 measuring 30 mm or longer increased the risk of TLF; 2) this association was not observed for any of the secondary endpoints analyzed; 3) complete revascularization was a protective factor for TLF; and, 4) in addition to the LSG, other independent predictors of TLF included restenosis treatment, previous heart failure, and severe clinical conditions (acute pulmonary edema, cardiogenic shock, or successfully resuscitated cardiac arrest).
Long DES2 studies can be classified into two groups: 1) single-cohort studies evaluating the success rates of implantation and the performance of different DES2, and 2) multiple-cohort studies comparing the outcomes of short versus long DES2.
4.1. Single-cohort Studies
Numerous retrospective and prospective single-cohort studies have been published [22-36]. The most commonly analyzed outcomes include TLF rates at one year (4.2 - 9%) [22-29] and at two years (5%) [30], and the composite outcome of CVD, myocardial infarction, and TLR at one year (2 - 3%) [31, 32], at two years (5.4 - 7.6%) [33, 34], and at three years (10.4%) [35].
Overall, an analysis of the data from these studies shows high success rates for the implantation of the evaluated long DES2 (92 - 100%) [22, 24-31, 33, 36]. Similarly, their performance, as assessed by different outcomes during varying follow-up periods, can be considered positive.
4.2. Multiple-cohort Studies
The most important multiple-cohort studies on long DES2 are summarized in Table 3. Conflicting results are observed; the first five studies reported favorable results [9-13], two presented partially favorable results [14, 15], and the last two reported unfavorable results for the considered long DES2 [16, 17]. In the studies by Honda et al. [14] and Hiromasa et al. [15], the intermediate groups (stent lengths of 20 - 50 mm and 23 - 46 mm, respectively) showed comparable results to those of cohorts with shorter stent lengths. Only the groups with the longest DES2 showed significantly higher endpoint rates. Consequently, the results of these studies were considered partially favorable.
The differences among these studies are striking, mainly due to the lack of standardized criteria for defining long stents, making it difficult to compare results and draw definitive conclusions. These cutoff values ranged from longer than 30 mm [13] to greater than 50 mm [14]. Hiromasa et al. [15] and Chandrasekhar et al. [16] used tertiles and quartiles, respectively, as cutoff values. Only Kong et al. analyzed the results based on the stratified lengths of the DES2 and determined the criterion for the cutoff measurement (greater than 40 mm) by clinical relevance [17]. All others have used “arbitrary” values, as in this manuscript. At the time this study was planned, the longest available DES2 was 38 mm in length. By authors’ consensus, a length of 30 mm or greater was chosen as the definition of a long stent to ensure a reasonable number of patients in the LSG. Similarly, the authors chose not to include individuals who received intermediate-length DES2 (21 - 29 mm) to allow a direct comparison between long and short DES2.
The three-year TLF rates observed in this study were 16.7% in the LSG and 10.4% in the SSG (p = 0.01). Excluding the most severe patients (acute pulmonary edema, cardiogenic shock, or successfully resuscitated cardiac arrest), the primary endpoint rates were 14.8% and 9.3%, respectively (p = 0.026). It is important to note that this study included complex participants with comorbidities that are typically considered exclusion criteria. These conditions may have contributed to the increased outcome rates in both groups.
| Author et al.\Year\Refs. | Design | Groups (mm) | n | Primary endpoint | t |
Primary endpoint (%) |
p |
|---|---|---|---|---|---|---|---|
| Choi [9] 2014 |
Prospective, observational, eight centers |
< 32 | 1733 | Death + MI + TVR |
3 | 9.5 | 0.089 |
| ≥ 32 | 378 | 10.9 | |||||
| Konishi [10] 2016 |
Prospective, observational, single center | ≤ 32 | 186 | Death +ACS + TVR |
3.6 | 13.9 | 0.24 |
| > 32 | 110 | 9.1 | |||||
| Bouras [11] 2017 |
Six trials pooled analysis |
> 24 a < 35 | 482 | TLF | 1 | 10 8.9 |
0.63 |
| ≥ 35 | 323 | ||||||
| Yano [12] 2018 |
Retrospective, observational, two centers | < 15 | 138 | CVD + MI + TVR + ST |
2 | 4.4 | 0.402 |
| 15 - 23 | 210 | 3.3 | |||||
| 24 - 32 | 190 | 4.7 | |||||
| > 32 | 192 | 4.7 | |||||
| Soontorndhada [13] 2020 |
Retrospective, observational, single center |
≤ 30 | 174 | CVD + MI + stroke + ST + TLR |
2.7 | 40.2 | 0.57 |
| > 30 | 96 | 43.8 | |||||
| Honda [14] 2016 |
Retrospective, observational, single center |
< 20 | 745 | TLR | 1.9 | 6 | 0.001 (between >50 mm and each of the shorter groups) |
| 20 - 50 | 758 | 7.2 | |||||
| > 50 | 166 | 13.5 | |||||
| Hiromasa [15] 2017 |
Retrospective, observational, single center |
8 - 23 | 382 | TLR | 3 | 6.6 | 0.001 (between 46 - 204 mm and each of the shorter groups) |
| 23 - 46 | 312 | 8 | |||||
| 46 - 204 | 313 | 18.8 | |||||
| Chandrasekhar [16] 2018 |
Fourteen trials registry | 8 - 18 | 782 | MACE | 3 | 9.2 | <0.0001 |
| 18 - 24 | 1706 | 11.1 | |||||
| 24 - 36 | 1329 | 14.4 | |||||
| ≥ 36 | 1586 | 19.6 | |||||
| Kong [17] 2021 |
Five registries pooled analysis | ≤ 40 | 8035 | TLF | 2 | 4.6 | <0.001 |
| > 40 | 1182 | 8.6 |
The higher rate of TLF in the LSG was attributed to the higher prevalence of patients undergoing procedures for restenosis treatment and the longer implanted DES2. These variables showed statistically significant differences between the two cohorts and were confirmed as independent predictors of TLF in the multivariate analysis.
Among the aforementioned studies, only two evaluated the predictors of TLF using multivariate analysis. Bouras et al. identified the number of affected vessels and previous myocardial infarction [11]. In the GRAND-DES registry, the predictors included LSG (greater than 40 mm), age, diabetes mellitus, peripheral arterial disease, chronic kidney disease, previous myocardial infarction, heart failure, cerebrovascular disease, left main coronary artery lesion, restenosis treatment, and left ventricular dysfunction [17]. Our data highlighted two distinct factors: complete revascularization as a protective feature and severe clinical conditions as a negative predictor. However, the former has already been shown to improve prognosis in patients with both chronic coronary disease [37] and acute myocardial infarction [38], whereas mortality progressively increases with the severity of cardiogenic shock and the occurrence of cardiac arrest [39, 40].
4.3. Clinical Implications
The use of long stents is becoming increasingly common in Interventional Cardiology due to the need to treat more complex lesions. Studies comparing the implantation of shorter overlapping stents with procedures using a single long DES2 to treat long lesions have not found significant differences in primary outcomes [41-43]. However, PCI with single longer stents showed significantly shorter procedure times [41, 44-46], reduced fluoroscopy time [41, 44, 46, 47], reduced contrast use [41, 43-46], and reduced costs [41]. Since long stents offer all these advantages, with outcomes at least comparable to those of shorter overlapping stents, their utility in daily clinical practice is obvious.
This study supports the findings of Chandrasekhar et al. [16] and Kong et al. [17], both of whom reported unfavorable outcomes for long DES2. Given the increasing use of these stents, further advances in stent technology, such as improvements in strut thickness and composition, polymers, drugs, and drug elution, are still needed to improve long-term outcomes.
4.4. Limitations
This study has inherent limitations related to its methodology, including the potential for different distributions of clinical and angiographic characteristics between groups and the influence of unmeasured confounders.
The rate of loss to follow-up was higher than that reported in all analyzed studies providing this information.
Current guidelines recommend prasugrel or ticagrelor as the preferred P2Y12 receptor antagonists for acute coronary syndromes [48]. Similarly, radial access should be preferred over femoral access [48, 49]. However, in this study, 63.4% of participants received clopidogrel, and 92.4% underwent PCI via femoral access, which may increase the risk of ischemic and hemorrhagic complications, respectively.
Lesion lengths and vessel diameters were assessed through a visual assessment of coronary angiography, and the SYNTAX score was not calculated for each patient. Left ventricular ejection fraction was assessed by ventriculography rather than echocardiography.
Intravascular imaging was not available despite substantial evidence of its benefit in reducing adverse outcomes [50].
Finally, a significant percentage of patients (20.4%) received different stents, which precludes specific conclusions regarding DES2 characteristics, such as strut thickness, polymer type, or eluted drug.
CONCLUSION
Despite technological advances, the use of DES2 measuring 30 mm or longer has been associated with a higher TLF rate. Therefore, the implantation of longer DES2, compared with DES2 measuring 20 mm or shorter, significantly impacted long-term clinical outcomes related to TLF, while MACE, safety, and efficacy outcomes were not affected.
Moreover, complete revascularization was identified as a protective factor for TLF. In addition to the LSG, other independent predictors of TLF included restenosis treatment, previous heart failure, and severe clinical conditions (acute pulmonary edema, cardiogenic shock, or successfully resuscitated cardiac arrest).
AUTHORS’ CONTRIBUTION
F.T.U: Contributed to the study concept and design, data collection, data analysis and interpretation, writing of the paper, and final approval; F.K.B. P.U: Contributed to writing the paper and final approval; A.P.M.K: Contributed to the study concept and design, data collection, data analysis and interpretation, and final approval; A.L.T. M.F., J S.B., T.A.F, and R.M.: Contributed to the data collection and final approval; Ê.E.G: Contributed to the data analysis and interpretation, writing of the paper, and final approval.
LIST OF ABBREVIATIONS
| 95%CI | = 95% Confidence Interval |
| ACS | = Acute Coronary Syndrome |
| APE | = Acute Pulmonary Edema |
| BARC | = Bleeding Academic Research Consortium |
| CABG | = Coronary Artery Bypass Grafting |
| CS | = Cardiogenic Shock |
| CVD | = Cardiovascular Death |
| DAPT | = Dual Antiplatelet Therapy |
| DES2 | = Second-generation Drug-eluting Stents |
| HR | = Hazard Ratio |
| LSG | = Long Stent Group |
| MACE | = Major Adverse Cardiovascular Events |
| MI | = Myocardial Infarction |
| PCI | = Percutaneous Coronary Intervention |
| SD | = Standard Deviation |
| SHR | = Subdistribution Hazard Ratio |
| SRCA | = Successfully Resuscitated Cardiac Arrest |
| SSG | = Short Stent Group |
| ST | = Definite or Probable Stent Thrombosis |
| TLF | = Target Lesion Failure |
| TLR | = Target Lesion Revascularization |
| TVR | = Target Vessel Revascularization |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The Research Ethics Committee of the Universidade Regional de Blumenau approved the research project on April 15, 2022, under substantiated approval number 5.353.025. It was duly registered in Plataforma Brasil, Blumenau, Brazil. All phases of the study were conducted in accordance with the Resolution 466/2012 of the Brazilian National Health Council.
HUMAN AND ANIMAL RIGHTS
All participants were enrolled in accordance with the ethical standards set by the committee responsible for human experimentation and in compliance with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
A waiver for the free and informed consent form was granted for this study as all data had already been collected, and the study was conducted by reviewing a pre-existing database.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of this article are available in the Zenodo Repository at https://zenodo.org/records/14861749, reference number 14861749, version v1, 2025”.
ACKNOWLEDGEMENTS
The authors would like to express their gratitude to Dr. Professor Márcia Olandoski for her invaluable guidance in data analysis and for her contributions to the conclusion of this study.


