All published articles of this journal are available on ScienceDirect.
Primary Non-adherence to Treatment with New Oral Anticoagulants: The Results of a Prospective Observational Study «ANTEY»
Abstract
Aim:
To assess the main characteristics of patients with non-valvular Atrial Fibrillation (AF) who are initially non-adherent to New Oral Anticoagulants (NOAC), and to identify factors associated with this version of non-adherence.
Materials and Methods:
The ANTEY study included 201 patients with non-valvular AF, who had indications and without contraindications for NOAC treatment. The patients had previously been advised to take oral anticoagulants but they did not comply with all medical recommendations. The observation period was 1 year, during which 2 in-person visits were performed: an inclusion visit (V0) and a visit (V1), as well as 1 telephone contact/follow up (FU); the interval between contacts was 6 months. All patients were recommended to take the NOAC by decision of the physician. During the V0, V1 and FU visits, the “National Society for Evidence-Based Pharmacotherapy (NSEPh) Adherence Scale” questionnaire was used to assess overall adherence and associated factors. 15 (7.5%) patients had not started NOAC therapy by the end of the study (primary non-adherent patients). Their characteristics are analysed in this work.
Results:
The main reasons for primary non-adherence to NOAC were high cost (33.3%), fears of adverse effects (AE) (33.3%), doubts about the need for treatment (13.3%) and the complex therapy regimen (13.3%). In the group of primary non-adherent patients in comparison with the rest of the patients there were significantly more patients with 1 point according to CHADS2VASc (20% and 2.2%, respectively, p = 0.029) and patients with 3 points according to HAS-BLED (33.3% and 9.1%, respectively, p = 0.006); they took antiplatelet drugs more often 73.3% versus 21.5%, respectively (p = 0.001). Full employment at work (OR = 5.2; CI95% [1.5; 18.1], p = 0.009), history of quitting smoking (OR = 5.1; CI95% [1.5; 17.0], p = 0,008), the presence of any pharmacotherapy AE (OR = 4.0; CI95% [1.01; 16.0], p = 0.048) increased the chance of primary non-adherence to NOAC by 4-5 times.
Conclusion:
The most vulnerable in relation to initiation of NOAC therapy for the prevention of thromboembolic complications in AF are those patients who continue to work or have any pharmacotherapy AE. The leading factors preventing the initiation of NOAC administration are their high cost, fear of the development of AE from the therapy, and patients’ doubts about the need for treatment with these drugs. The clinical trial registration number is NCT 03790917.
1. INTRODUCTION
The World Health Organisation report on adherence to treatment in patients with chronic noncommunicable diseases stated that successfully resolving adherence issues could be a much more promising and effective direction than the development of new drugs and other therapies [1]. It has been shown that patient adherence can vary depending on the stage of therapy [2-5]. Several phases of adherence have been identified, corresponding to different stages of treatment: initiation of therapy, implementation and long-term adherence to therapy during the entire treatment period (persistence) [6, 7]. The initiation phase of therapy is considered particularly important in shaping the patient’s attitude towards adherence to the Physician’s Recommendations (PR). Terms such as “primary non-adherence” or “complete non-adherence” are used to denote the patient’s refusal of the recommended treatment, while “non-persistence” means a refusal to continue treatment that had already been started [8-10].
It is known that the most vulnerable patients in terms of adherence to treatment are those with few or asymptomatic chronic diseases or if there is no proper monitoring of the indicators of the effectiveness of the therapy. These criteria fully correspond to those patients with non-valvular Atrial Fibrillation (AF) receiving therapy with New Oral Anticoagulants (NOAC). Refusal to take these drugs leads to a high risk of thromboembolic complications. Determination of the main characteristics of patients with non-valvular AF who refuse to start taking NOAC and identifying factors that prevent the initiation of NOAC treatment is an important and urgent task.
The aim of this work was to assess the main characteristics of patients with non-valvular AF who were initially non-adherent to NOAC to identify the factors associated with this version of non-adherence.
2. MATERIALS AND METHODS
The ANTEY study (Assessment of Adherence to New Oral Anticoagulants in Atrial Fibrillation Patients Within the Outpatient Registry) is registered on www.clinicaltrials.com (NCT03790917) and was part of the PROFILE outpatient prospective registry that was developed on the basis of a specialised cardiology department. The study protocol was approved by the Independent Ethics Committee, all patients signed an informed consent form for the processing of personal data and participation in the ANTEY study.
Inclusion criteria - age over 18 years, the presence of indications for taking NOAC in accordance with the current clinical guidelines [11], the absence of contraindications for taking NOAC in accordance with the official instructions for these drugs [12].
To participate in the study, 225 patients with non-valvular AF were invited from the PROFILE registry, 24 patients refused to participate; a total of 201 people were included (118 men (58.7%) and 83 women (41.3%), the average age was 69.2 ± 8.9 and 73.2 ± 7.9 years, respectively). The patients had previously been advised to take oral anticoagulants but they did not comply with all medical recommendations.
The study protocol included 2 face-to-face contacts between the patient and the physician (visits V0 and V1) and 1 telephone contact (Follow-Up (FU)), the intervals between contacts were 6 months; the observation period lasted 1 year. At visit V0, medical history data, socio-demographic indicators, risk factors, comorbidities, scores according to the CHADS2VASc and HAS-BLED scales, prior Oral Anticoagulant Therapy (OAC), information on Adverse Effects (AE) of OAC and any prior history of pharmacotherapy AE were specified.
On visit V0, adjustment of the OAC therapy was proposed, which was carried out according to one of three possible scenarios.:
1) The first option was used in patients receiving NOAC, when the physician could continue therapy with the same NOAC, and if necessary, adjust its dose, frequency of administration according to current clinical guidelines or replace it with another NOAC.
2) The second option was used in patients who received warfarin, which was replaced by a NOAC. At the time of inclusion into the study, warfarin was taken by 21 (10.4%) people. Among the patients who took warfarin, in 4 people INR was monitored with the regularity of 1 time per month, in 11 people INR was controlled with the regularity of 1 time every 3 months, in 4 patients this indicator was analyzed 1 time every six months, and in 2 people INR was not controlled.
When analyzing the relative time of INR in the target range (Time in Therapeutic Range - TTR), it was found that in 2 people, the target values of INR were not achieved (TTR=0%), in 6 people, the TTR indicator varied from 29% to 50%. Only in 7 (36.8%) of 19 patients, the TTR was higher than 70% (the preferred value of the time of INR within the target range). However, all these 7 patients experienced a number of inconveniences when taking warfarin (the need for regular monitoring of INR, compliance with the diet, and the exclusion of taking a number of medications). In 4 cases, the TTR was not calculated by the attending physician.
3) The third option was to prescribe a NOAC to patients who had not received any OAC before the V0 visit.
Therefore, NOAC were recommended for all patients at visit V0. After 6 months (visit V1), the NOAC could be changed for another one or for warfarin according to doctor’s decision.
At visits V0 and V1, the physician informed the patient about the need to take NOACs to prevent thromboembolic complications of AF, about the importance of taking the drug regularly, possible AE, the need to contact a physician in case of bleeding, planned surgical interventions and when other specialists prescribe drugs. On visits V0, V1 and FU, the special questionnaire “National Society for Evidence-Based Pharmacotherapy (NSEPh) Adherence Scale” was used to assess overall adherence and related factors [10]. According to NSEPh, potential (at visit V0) and actual (at visits V1 and FU) adherence was divided into 4 types: complete adherence for patients who took the NOAC in strict accordance with the physician’s recommendations (PR), partial adherence for patients who violated the NOAC regimen (skipping, changing the dose, frequency, etc.), partial non-adherence for patients who started taking the NOAC, but who then discontinue treatment, and complete non-adherence/primary non-adherence for patients who refuse to take the NOAC during a visit with the physician. During one year of follow-up, patients who had not started taking the OAC were identified.
Statistical data processing was performed using the SPSS Statistics 23.0 statistical software package (IBM, USA). Standard descriptive statistical methods were used: mean values and standard deviations - for normally distributed quantitative variables; percentages - for qualitative variables (the normality of distribution was estimated using the Shapiro-Wilk W test). A comparison of qualitative variables was carried out using Pearson's x2 test. To determine the significant factors associated with complete non-adherence, a logistic regression model was constructed, the Odds Ratio (OR) was calculated with a 95% confidence interval - CI95% using the Mantel-Henzel statistics for dichotomous variables. Statistical significance was set at p <0.05.
3. RESULTS
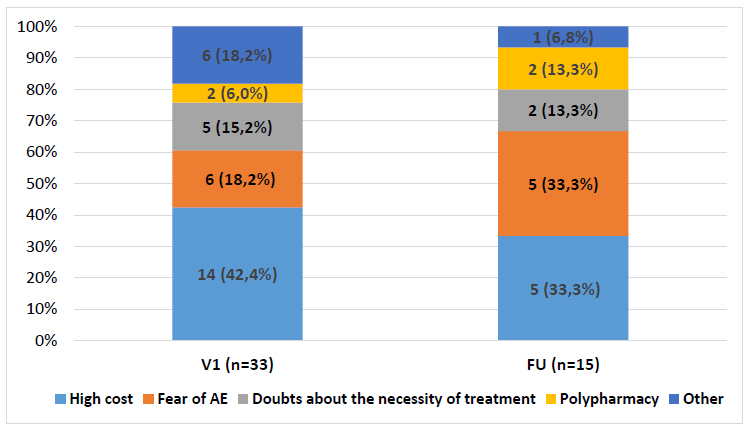
At the time of V1 behaviour (6 months of follow-up), 33 (16.4%) patients had not started taking the prescribed NOAC. The main reasons for the refusal to take the NOAC were the high cost, fear of developing adverse effects (AE), and doubts about the need to take the drug (Fig. 1).
After 1 year of observation (contact FU), 18 out of 33 patients started taking NOAC, and 15 (7.5%) patients remained in the primary non-adherence category. The main characteristics of these 15 patients were analysed in this work.
The main comparative characteristics of primary non-adherent and all other patients are present in Table 1.
| - |
Primary Non-Adherent Patients, n=15 |
All Other Patients, n=186 |
Statistical Significance p |
|---|---|---|---|
| Men, n (%) | 10 (66.7) | 108 (58.1) | 0.52 |
| Age, M±SD | 66.7±9.5 | 71.5±8.6 | 0.06 |
| Smoking, n (%) | |||
| Current smokers | 1 (6.6) | 13 (7.1) | >0.05 |
| Former smokers | 7 (46.7) | 31 (17.0) | 0.019* |
| Never smokers | 7 (46.7) | 138 (75.9) | 0.019* |
| Alcohol consumption (>8 drinks per week) (HAS-BLED score), n (%) | 2 (13.3) | 3 (1.6) | 0.005* |
| Employment, n (%) | |||
| Permanent employees | 7 (46.7) | 39 (21.0) | 0.037* |
| Casual employees | 2 (13.3) | 14 (7.5) | >0.05 |
| Non-employees | 6 (40.0) | 133 (71.5) | 0.037* |
| Retirees, n (%) | 12 (80.0) | 168 (90.3) | 0.21 |
| Hypertension, n (%) | 12 (80.0) | 178 (95.7) | 0.01* |
| AMI, n (%) | 5 (33.3) | 53 (28.5) | 0.69 |
| Chronic heart failure, n (%) | 5 (33.3) | 94 (50.5) | 0.20 |
| Diabetes mellitus, n (%) | 4 (26.7) | 56 (30.1) | 0.78 |
| CHA2DS2-VASc score=1, n (%) | 4 (26.7) | 3 (1.6) | 0.029* |
| HAS-BLED score ≥3, n (%) | 6 (40.0) | 18 (9.7) | 0.006* |
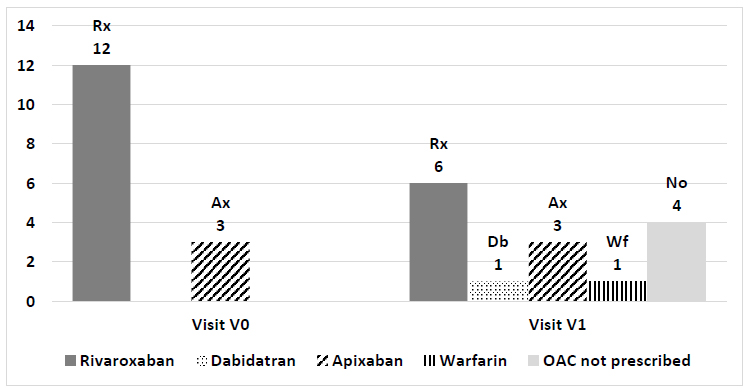
Note: Rx - rivaroxaban, Ax - apixaban, Db - dabigatran, Wf - warfarin, No - NOAC not prescribed (patients persistently refused to take any OAC).
All 15 patients, when included in the ANTEY study, did not take OAC; one of them had previously taken warfarin, which was discontinued due to moderate bleeding. In comparison with the rest of the patients in the cohort, these patients took antiplatelet agents more often as antithrombotic therapy, 73.3% and 21.5%, respectively, p = 0.001. The main reasons for non-adherence to the NOAC were fears of AE and the high cost of the drug (Fig. 1).
The physician’s anticoagulant prescriptions during visits V0 and V1 are shown in Fig. (2). At the visit, V1 warfarin was recommended to the patient who refused to take NOAC. Four primary non-adherent patients refused to take any OAC (NOAC not prescribed).
In this group, in comparison with the rest of the patients, there were more who were working (60% and 28.5%, respectively, p = 0.025) and patients who quit smoking (46.7% and 17.0%, respectively, p = 0.015) and more patients who abused alcohol (13.3% and 1.6%, respectively, p = 0.005). The groups that were compared did not differ in the number of smoking patients (7% in each group). In primary non-adherent patients, arterial hypertension was statistically significantly less frequent (80% and 95.7%, respectively, p<0.05) and idiopathic AF was more frequent (13.3% and 2.2%, respectively, p = 0.014). The presence of pharmacotherapy AE in the medical history was more often detected in primary non-adherent patients compared with the rest of the patients (26.4% and 6.25%, respectively, p = 0.006). In addition, in the group of primary non-adherent patients, there were significantly more patients with 1 point according to CHADS2VASc (20% and 2.2%, respectively, p = 0.029) and patients with 3 points according to HAS-BLED (33.3% and 9.1%, respectively, p = 0.006).
Table 2 shows the factors for which a significant relationship with complete non-adherence was found.
| Factor | OR | CI95% | р |
|---|---|---|---|
| Presence of AH | 0.18 | [0.04; 0.77] | 0.038 |
| Idiopathic AF | 7.00 | [1.17; 41.85] | 0.07 |
| Taking antiplatelet agents | 7.80 | [2.13; 28.69] | 0.0006 |
| Alcohol abuse (according to the HAS-BLED scale) | 9.68 | [1.44; 28.69] | 0.046 |
When constructing a logistic regression model, it was revealed that the significant factors associated with a four to fivefold increase in the patient’s chances of complete non-adherence and refusal of the NOAC are full employment at work (OR = 5.2; CI95% (1.5; 18.1), p = 0.009), history of quitting smoking (OR = 5.1; CI95% (1.5; 17.0), p = 0.008), the presence of any pharmacotherapy AE (OR = 4.0; CI95% (1.01; 16.0), p = 0.048).
4. DISCUSSION
It is known that adherence can change at different stages of treatment under the influence of various factors [2-5]. The initiation phase of therapy is particularly vulnerable to adherence to the physician’s prescription. At this stage of treatment, one often notes the patient’s cautious attitude towards a new prescription, replacement of the previous treatment with an alternative one, and fear of the development of an AE for a drug that has not been previously taken. The refusal to start taking a recommended drug reflects the essence of the problem of unsatisfactory adherence, which was formulated by the American paediatric surgeon C.E. Koop: “Drugs don't work in patients who don't take them” [13, 14]. In addition, it must be emphasised that refusal to start treatment, regardless of the reasons which cause it, is always a variant of intentional non-adherence (as opposed to unintentional violation of medical prescriptions, most often due to forgetfulness).
When treating patients with non-valvular AF who have indications for NOAC therapy, the attending physician must pay attention to the fact that working patients without arterial hypertension, possibly with idiopathic AF, alcohol abuse, and who quit smoking for various reasons are in the risk group for full non-adherence and refusal to start taking NOACs. This group is more likely to include patients with a very low risk of thromboembolic complications on the CHADS2VASC risk scale. The results of other studies confirm that a more severe clinically pronounced course of the disease has a positive effect on patient adherence and prevention measures and prevents the clinical inertia of doctors with regard to prescribing the therapy, dose titration, and patient motivation to follow the recommendations [15-18].
According to the medical history data of primary non-adherent patients, it turned out that only one of them had previously taken warfarin, which resulted in moderate bleeding. At the time of the ANTEY study, the mandatory health insurance system in Russia provided patients with non-valvular AF with warfarin only, not the NOAC. The NOAC had to be purchased at the patient's own expense. Among the main reasons for refusing to take NOAC were their high cost and the fear of AE. Probably free distribution of NOAC will reduce the significance of the high price as a cause of primary non-adherence. Patients' fear of AE can be reduced by providing patients with complete information about the importance and necessity of these drugs to prevent severe complications of AF.
The rate of primary non-adherence/total non-adherence in the ANTEY study (7.5%) coincides with the results of a prospective cohort study conducted in Spain, in which upon prescribing of the NOAC, 5.6% of patients did not receive even the first dose of the drug, i.e. they turned out to be primarily non-adherent [9].
It must be noted that after six months of follow-up, the number of primary non-adherent patients was 16.4%, which is consistent with the results of a study conducted by T.C. Cheetham et al., which found 15.6% of primary non-adherent patients during the first prescription of a statin. This study also showed that primary non-adherent patients are generally younger and less likely to require urgent care [15].
The physician plays a very important role in resolving the problem of poor adherence. Due to the repeated physician’s instruction of the patient, which included an explanation of the need for continuous use of NOAC for AF, the number of primary non-adherent patients after 1 year of follow-up decreased by half. Obviously, the frequency and regularity of patient visits to the physician play an important role in the timely evaluation of effectiveness, in the adjustment of the therapy, as well as in additional instructions for the patient when unsatisfactory adherence is detected. Some researchers even distinguish this indicator as a special type of adherence - attendance [19, 20]. Thus, a regular visit to a physician with coronary heart disease doubled adherence to treatment [20]. According to a Japanese study involving 545 women with osteoporosis, it was found that the patient’s decision to start treatment with a new drug was influenced by awareness of the disease and its complications, regular visits to the physician, absence of polypharmacy and a comprehensive treatment regimen at the time of a new prescription [3].
Primary patient non-adherence is a worrying indicator when considering the serious health and life implications of a complete refusal to take necessary drugs. Given the intentional nature of this type of adherence violation and its formation in the early stages of treatment, it is obvious that the most effective measures to prevent it are the establishment of a partnership between the physician and the patient with the development of a clear motivation in the patient to take the recommended drugs. Explanations by the physician about the type of therapy prescribed, its effectiveness in preventing severe and often life-threatening complications, and the need and features of monitoring safety indicators, can increase the patient’s knowledge of the need for treatment, the ability to monitor its safety, and generally increase adherence to the physician’s recommendations and treatment.
5. LIMITATIONS
Due to the small size of the group, a variant of the chisquare statistic, the exact Fisher criterion, was used; OR and 95% CI were also calculated. Despite the small number of primary non-adherent patients, it was possible to construct a logistic regression model of relatively satisfactory quality (Nagelkerke R Square is 0.3) and to obtain significant β-coefficients and Exp (β) values (OR) for several independent predictors of the model.
CONCLUSION
The results of the study showed that those most vulnerable in regard to the initiation of NOAC therapy for the prevention of thromboembolic complications in AF are patients who continue to work, those with idiopathic AF and a low risk of thromboembolic complications, those who have no experience of taking OACs, those who are without arterial hypertension, alcohol abusers, and those already taking antiplatelet drugs. The leading factors preventing the initiation of NOAC administration are their high cost, fear of the development of AE from the therapy, and patients’ doubts about the need for treatment with these drugs.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study has been approved by the Independent ethics committee of the National State Research Center for Therapy and Preventive Medicine, with registration number 05-01 /17.
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
The authors confirm that (a) the laws, regulations, and code(s) of ethics applicable at the site where the Observational Study is conducted, including without limitation the Federal Law dated 12th April 2010 No. 61-FZ ―On circulation of medicines and Federal Law dated 21st November 2011 No. 323-FZ ―On fundamentals of health protection of citizens in the Russian Federation; (b) orders and mandates of the relevant authorities and local ethics committees.
CONSENT FOR PUBLICATION
All patients signed an informed consent form for the processing of personal data and participation in the ANTEY study.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of our article is available in the Zenodo repository at: DOI: 10.5281/zenodo.5040652, https://zenodo.org/record/5040652#.YWPdvdpBw2w
FUNDING
This research was funded by AG BAYER with the reference number IIR-RU-2016-3743.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


