All published articles of this journal are available on ScienceDirect.
Risk of Cerebral Embolization with Caseous Calcification of the Mitral Annulus: Review Article
Abstract
Background:
Caseous calcification of the mitral annulus (CCMA) is believed to have a benign prognosis. Several authors have recommended conservative management in asymptomatic patients. However, the prevalence of cerebrovascular events (CVE) in patients with CCMA has never been evaluated before. The aims of this study are to investigate whether patients with CCMA are at increased risk of cerebral embolization, and to determine whether elective surgical resection of CCMA should be considered to prevent a cardioembolic stroke.
Methods:
A comprehensive literature search was obtained from MEDLINE via PubMed.gov, ScienceDirect.com, and Google Scholar using the following search queries: caseous calcification of the mitral annulus, intracardiac pseudotumor, mitral annular calcification, and cardioembolic stroke.
Results:
From our initial search that yielded 1,502 articles, we identified a total of 130 patients with CCMA reported in 86 publications. Literature review revealed that the prevalence of CVE associated with CCMA is 19.2% (25 of 130) which is significantly higher than the prevalence of CVE reported with mitral annular calcification (MAC), 11.8% (214 of 1818) (range 4.8% to 24.1%) (P = 0.01796) (odds ratio = 1.78; 0.95 confidence interval = 1.1278 – 2.8239). Only four of 25 (16.0%) patients with CCMA who suffered a CVE had history of atrial fibrillation (AF).
Conclusion:
Based on our review, it would be reasonable to consider elective surgical resection of CCMA in asymptomatic patients who are good surgical candidates, because patients with CCMA may be at increased risk of embolic strokes, which are unrelated to AF.
INTRODUCTION
Approximately one-fifth of all ischemic strokes are cardioembolic. Several cardiac conditions have been identified as potential sources of embolism. Atrial fibrillation (AF) is the most common cause of cardioembolic stroke. Other major potential sources are: mitral stenosis, prosthetic valves, infective endocarditis, marantic endocarditis, recent myocardial infarction, dilated cardiomyopathy, left ventricular aneurysm, and intracardiac tumors. Mitral annular calcification, caseous calcification of the mitral annulus, calcific aortic stenosis, patent foramen ovale, atrial or ventricular septal defects, and atrial septal aneurysm may also be associated with cardioembolism [1]. Mitral annular calcification (MAC) is a common echocardiographic finding, mainly in older patients. The association between MAC and atrial fibrillation, cerebral embolization, acquired mitral stenosis, hypertension, hyperlipidemia, diabetes, coronary artery disease, aortic atheroma, and diffuse atherosclerosis has been well documented. According to several clinical and clinicopathologic studies [2-9], the risk of cerebrovascular events (CVE) in patients with MAC ranges from 4.8% to 24.1% (Table 1).
Caseous calcification of the mitral annulus (CCMA) is a rare variant of MAC, seen as a tumor-like echo-dense mass containing central areas of echolucencies at the posterior periannular region of the mitral valve on echocardiography [10]. Several authors have suggested that conservative management is recommended if the diagnosis of CCMA can be made by echocardiography in asymptomatic patients because CCMA is usually a benign condition [10-13]. However, the prevalence of CVE in patients with CCMA has never been evaluated before. The aims of this study are to investigate whether patients with CCMA are at increased risk of cerebral embolization, and to determine whether elective surgical resection of CCMA should be considered to prevent a cardioembolic stroke.
CVE = cerebrovascular event; MAC = mitral annulus calcification.
MATERIALS AND METHODOLOGY
A comprehensive literature search on all peer-reviewed studies published in English language was obtained from MEDLINE via PubMed.gov, ScienceDirect.com, and Google Scholar on landmark and related articles to investigate whether patients with CCMA are at increased risk of cerebral embolization. The search queries included: caseous calcification of the mitral annulus, intracardiac pseudotumor, mitral annular calcification, and cardioembolic stroke. To ensure that no potentially relevant articles were missed, the reference lists of all the articles included in the search were checked and cross-referenced.
Statistical Analysis
The Chi-squared test was used to determine whether there is a significant difference between the observed frequencies of cerebrovascular events in patients with CCMA compared to MAC. P values less than 0.05 were considered significant.
RESULTS
From our initial search that yielded 1,502 articles, we identified a total of 130 patients with CCMA that were reported in 86 English-language publications (see Appendix 1). These include 57 publications on caseous calcification of the mitral annulus, 14 articles on liquefaction necrosis of the mitral annulus, 7 articles on mitral annular calcification that included patients with CCMA, and 8 papers on intracardiac pseudotumors that were included because the patients had CCMA. In addition, 435 articles were reviewed to evaluate the prevalence of stroke in patients with MAC.
The first manuscript describing “caseation” of the calcified mitral ring was published in 1970, consisting of 7 cases of CCMA identified in an autopsy study by Pomerance [14] among 258 cases of MAC. Most of the publications on CCMA (74/86, or 86.0%) were single case reports. The three largest published series consisted of 19, 14 and 6 patients, respectively [10-12]. A total of 137 patients with CCMA were identified in these 86 publications, 130 of which were clinical cases, in addition to 7 postmortem cases reported by Pomerance [14].
The mean age of patients with CCMA was 69.5 years (range 23 to 92 years-old). Female gender predominated (94/130, or 72.3%). The majority of the 130 patients reported in clinical studies (111/130, or 85.4%) diagnosed to have CCMA were being evaluated because they were symptomatic. Only 14 (10.8%) of the 130 patients with CCMA were completely asymptomatic, and the clinical history was unknown in 5 (3.9%). The most common symptom at presentation was dyspnea on exertion or at rest (49/130 patients, or 37.7%). Twenty-two (44.9%) of the 49 patients presenting with dyspnea had moderate or severe mitral regurgitation (MR), and five (10.2%) had moderate or severe mitral stenosis (MS). Other symptoms included: cerebrovascular events (CVE), syncope, palpitations, and chest pain.
The prevalence of CVE in patients with CCMA was significant. Twenty-three of 130 (17.7%) patients presented with a stroke [10, 15-32], and 2 additional patients being managed conservatively had a CVE during follow-up [11, 33] (Table 2). Thus, the total number of patients with a confirmed diagnosis of CCMA who had a CVE was 25 of 130 (19.2%). Atrial fibrillation (AF) was present in 21 (16.2%) of the 130 patients. However, only 4 of the 25 (16.0%) patients with a CVE had documented AF, whereas the majority (21/25, or 84.0%) of CCMA patients with a CVE did not have AF (Table 2). Harpaz and associates [10] reported AF in 2 of their 19 patients, and a CVE in 5 of their patients. It is not clear from their article whether the two patients with AF presented with CVE or not.
Associated risk factors included hypertension, present in 61.5% (80/130) patients, dyslipidemia in 23.8% (31/130), diabetes mellitus in 16.9% (22/130), and end-stage renal disease (ESRD) on chronic hemodialysis in 11.5% (15/130). However, the prevalence of these risk factors is likely to be higher, because the comorbidities were not mentioned in 24 patients.
The diagnosis of CCMA was made with transthoracic echocardiography (TTE) in approximately half the cases (64/130, or 49.2%), and by transesophageal echocardiography (TEE) in 9 additional patients. The typical appearance of CCMA on 2-dimensional echocardiography is a round, echodense mass mimicking an intracardiac tumor, containing central areas of echolucencies resembling liquefaction, located in the posterior aspect of the mitral annulus or periannular region [10]. The echodense mass was located in the posterior aspect of the mitral annulus in 124 patients (95.4%), and involvement of the base of the anterior leaflet was reported in 6 cases (4.6%). Additional diagnostic imaging studies, including cardiac computed tomography (CT) scan, and cardiac magnetic resonance imaging (CMRI) were necessary to establish the diagnosis of CCMA in 37 patients. In 18 (13.8%) patients the diagnosis of CCMA was not suspected from preoperative studies and was made intraoperatively.
Almost two-thirds of the patients (84/130, or 64.6%) reported in the literature were managed conservatively, including 9 patients who presented with a CVE. During follow-up, 3 medically treated patients had a CVE (2 of whom died), 2 had syncopal episodes and 4 patients died: 2 from a CVE, 1 had sudden death, and 1 from erosion of the CCMA mass into the circumflex artery.
Forty-two patients (32.3%) were referred to surgery, 5 of whom refused, thus only 37 of the 111 (33.3%) symptomatic patients underwent an operation. Only 10 (38.5%) of the 26 patients with a preoperative diagnosis of possible cardiac tumor established by TTE underwent resection. The most common indication for surgical treatment was the association of CCMA with severe mitral regurgitation in 22 patients and severe mitral stenosis in 5. The surgical procedure consisted of resecting the CCMA mass, combined with mitral valve replacement (MVR) in 24 patients and mitral annuloplasty in 6.
| Author | Year | Pts (n) | CVE | Rhythm | Treatment |
|---|---|---|---|---|---|
| Harpaz [10] | 2001 | 19 | CVE 5 | AF 2 NSR 17 | observation 16 + surgery 3 |
| Deluca [11] | 2008 | 14 | CVE 1 (at F/U) | AF 5 NSR 9 | observation 14 |
| Davidson [15] | 2006 | 1 | CVE (retinal embolus) | ? | surgery |
| Lubarsky [16] | 2007 | 1 | CVE | ? | observation |
| Poh [17] | 2007 | 1 | CVE | ? | surgery |
| Higashi [18] | 2010 | 1 | CVE | NSR | anticoagulation |
| Stamou [19] | 2010 | 1 | CVE (retinal embolus) | ? | surgery |
| Chevalier [20] | 2011 | 1 | CVE | ? | surgery |
| Gulati [21] | 2011 | 1 | CVE (TIA) | ? | refused surgery |
| Vijayan [22] | 2011 | 1 | CVE | NSR | anticoagulation |
| Akram [23] | 2012 | 1 | CVE | ? | observation |
| Chen [24] | 2012 | 1 | CVE | ? | observation |
| Matsuyama [25] | 2012 | 1 | CVE | NSR | observation |
| McKernan [26] | 2012 | 1 | CVE | ? | surgery |
| Motwani [27] | 2012 | 1 | CVE | ? | observation |
| Corre [28] | 2013 | 1 | CVE | NSR | anticoagulation |
| Kalayci [29] | 2013 | 1 | CVE | NSR | surgery |
| Pugliatti [30] | 2013 | 1 | CVE | NSR | observation |
| Mallisho [31] | 2014 | 1 | CVE | NSR | refused surgery |
| Sequeira [32] | 2014 | 1 | CVE | ? | surgery |
| Namura [33] | 1992 | 1 | CVE 1 (at F/U) | AF | observation |
AF = atrial fibrillation; CCMA = caseous calcification of the mitral annulus; CVE = cerebrovascular event; F/U = follow-up; NSR = normal sinus rhythm; Pts = patients; TIA = transient ischemic attack.
According to our review, 43.2% (16 of 37) of the surgical patients were ≥70 years-old patients, and only 7 (28.0%) of the 25 CCMA patients with a CVE underwent surgery, 5 of whom were ≥70 years-old. The overall surgical mortality was 8.1% (3 of 37) for all published CCMA cases, including 2 of 16 patients aged ≥70 years-old (12.5% mortality).
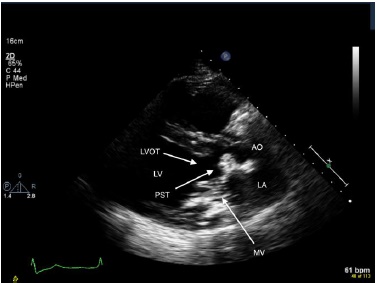
In addition to the literature search, we report 3 patients who were diagnosed to have CCMA intraoperatively. One patient was referred to surgery with a suspected diagnosis of left atrial myxoma; another patient with diagnosis of severe MS and heavy MAC; and another patient, who presented with TIA’s, was referred for resection of a subaortic mass that was attached to the anterior mitral annulus and was causing subtotal LV outflow tract obstruction (Fig. 1). On sectioning, the encapsulated mass contained a white toothpaste-like caseous material in all 3 patients.
DISCUSSION
Caseous calcification of the mitral annulus (CCMA) is a rare variant of MAC, which is observed in 0.067% of all echocardiographic exams, and is present in 0.63% of all patients with MAC [10, 11]. The typical appearance of CCMA is an echo-dense mass containing central areas of echolucencies at the posterior periannular region of the mitral valve on echocardiography [10, 11]. Caseous calcification of the mitral annulus is frequently misdiagnosed as an intracardiac tumor [10]. Several authors have suggested that conservative management is recommended if the diagnosis of CCMA can be made by echocardiography in asymptomatic patients because CCMA is usually a benign condition [10-13].
However, the prevalence of CVE in patients with CCMA has never been evaluated before. Our comprehensive review of the literature revealed that the prevalence of cardioembolic events associated with CCMA is 19.2% (25 of 130) which is significantly higher than the prevalence of CVE reported with mitral annular calcification (MAC), 11.8% (214 of 1818) (range 4.8% to 24.1%) (P = 0.01796) (odds ratio = 1.78; 0.95 confidence interval = 1.1278 – 2.8239).
Furthermore, the prevalence of CVE in patients with CCMA may be underestimated, because the vast majority of articles on CCMA were single case reports with no follow-up data, or very brief periods of follow-up, and the longest follow-up recorded was 3.8 years [10], whereas articles on the risk of stroke in patients with MAC usually refer to an older population followed for longer periods, up to 8-years [6]. It is also quite possible that several cases reported in the literature as “MAC” during the 1980’s and 1990’s may have been CCMA, due to underdiagnosis of CCMA, as many cardiologists were not familiar with this condition [13]. In addition, improvements in echocardiography and widespread use of cardiac imaging modalities during the last decade have helped to uncover this rare variety of MAC, which has been greatly underappreciated, and even unrecognized in the past [10].
In several case reports, CCMA was directly linked to cerebral embolization [18-32]. Thorough review of all cases of CCMA presenting with a stroke excluded thrombus formation and other possible intracardiac source of embolism by TEE or during operation in all 25 cases. The possible mechanisms for cerebral embolization from CCMA lesions include: (1) spontaneous fistulization and embolization of caseous material that may leak directly into the left atrium or ventricle [18, 20]; (2) embolization of caseous necrotic debris from the CCMA lesion [25]; and (3) embolization of calcium and cholesterol particles [32].
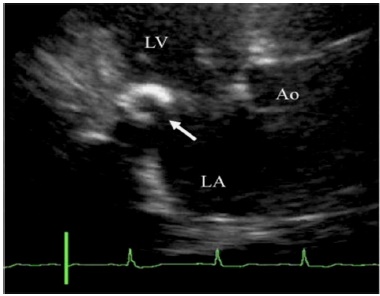
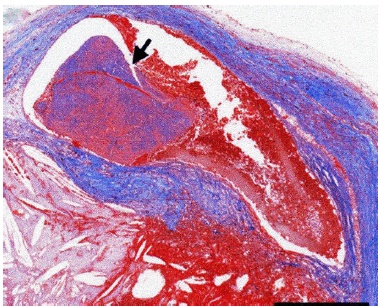
Higashi and associates [18] suggested that there was a causal relationship between the CCMA lesion and the stroke, because there was no evidence of extracranial atherosclerosis, and because they were able to demonstrate in one patient that the central cavity within the CCMA communicated with the left atrium (Fig. 2). The authors believed that caseous material leaked from the CCMA lesion into the systemic circulation and embolized to the brain. In another case report, Chevalier and colleagues [20] were able to confirm during surgery a direct communication between the CCMA and the left ventricle in a patient who presented after two strokes, and suggested that spontaneous fistulization is a possible mechanism of embolization of caseous material. Another two articles addressing the issue of possible coexisting extracranial carotid disease suggested that embolization originated from the CCMA lesion, and not from the carotid arteries. Matsuyama and coworkers [25] reported a case where caseous necrotic debris from the CCMA lesion had embolized to the brain and heart in a postmortem exam, in a patient without coexisting extracranial carotid disease (Fig. 3). Sequeira and associates [32] found bilateral branch retinal artery occlusion secondary to calcium and cholesterol emboli in the retinal vessels of a young patient on hemodialysis complaining of visual disturbances, in which the carotid ultrasound did not reveal any plaques within the internal carotid arteries.
In addition to these studies, Poh and co-workers [17] observed a sudden decrease in size of the left atrial mass on repeat echocardiograms in a patient who presented with an embolic stroke. This finding suggests that the CCMA lesion leaked caseous material into the systemic circulation, causing an embolic stroke. Deluca and associates [11] hypothesize that the reduction in size of the CCMA mass observed in 3 patients could be secondary to central liquefaction and gradual filtration of caseous material through microscopic leaks of the external wall.
One may argue that AF, which was present in 21 (16.2%) of 130 patients with CCMA could be a contributing factor for the increased prevalence of CVE in these patients. However, only 4 of 25 patients (16.0%) with CCMA who suffered a CVE had documented history of AF (Table 2). Thus, the available data shows that there is no correlation between AF and CVE in patients with CCMA, suggesting that the high prevalence of CVE was likely secondary to the CCMA, and not to the associated AF. In other words, patients with CCMA may be at increased risk of cerebral embolization which is not related to atrial fibrillation.
In summary, our study shows that 1 out of 5 patients with CCMA suffered embolic strokes. Patients with advanced age ≥70 years-old, female gender, and hypertension are even more likely to have cardioembolic strokes. Anticoagulation and antiplatelet therapy are ineffective to prevent a cardioembolic stroke in patients with CCMA, because the mechanism of embolization from CCMA lesions is unlikely to be related to thrombi. As an alternative, surgical treatment has been recommended by several investigators in patients who suffered a CVE.
THERAPEUTIC IMPLICATIONS
Many investigators have suggested that conservative management is justified if the diagnosis of CCMA can be made by echocardiography in asymptomatic patients [10-13]. However, there is no consensus on the optimal management for CCMA, as stated by Elgendy and Conti [13] in a recent review article. The authors suggest that, in most cases, conservative management for this lesion is sufficient, and that anticoagulation should be considered only in patients with CCMA who present with embolic manifestations [18, 22, 28]. In contrast, several authors recommend surgical intervention for associated mitral valvular dysfunction (either stenosis or regurgitation), embolic manifestations, or when it is impossible to rule out the possibility of a tumor.
The diagnosis of CCMA should be a major reason for concern because of the high prevalence of embolic stroke, which is likely attributed to embolization of caseous necrotic debris. Although anticoagulant therapy has been considered for some of these patients, anticoagulation is ineffective to prevent a cardioembolic stroke in these patients. As suggested by Corre and colleagues [28], further studies are needed to resolve the optimal treatment to decrease the risk of embolization in patients with CCMA. Thus, it is important to differentiate CCMA from MAC. Although MAC can be a nidus for thrombus formation, and has been described as a possible mechanism of stroke, the mechanism of embolization from CCMA lesions is unlikely to be related to thrombi, because review of all cases of CCMA presenting with a stroke revealed no thrombus formation and no other intracardiac source of embolism.
This may be an argument in favor of elective surgical resection of the CCMA lesion in asymptomatic patients who are good surgical candidates, because anticoagulation and antiplatelet therapy are probably ineffective to prevent a cardioembolic stroke in patients with CCMA. This suggestion is also based on the fact that the reported surgical mortality was quite acceptable (8.1%), especially considering that 43.2% (16 of 37) of the surgical patients were ≥70 years-old.
CONCLUSION
Despite the fact that several reports suggest that CCMA is a benign condition, patients with CCMA may be at increased risk of embolic strokes, which are unrelated to atrial fibrillation. The high prevalence of cerebral embolization from CCMA is likely attributed to spontaneous fistulization and embolization of caseous necrotic debris. Most authors agree that surgical treatment is indicated in symptomatic patients with CCMA associated with severe mitral regurgitation or stenosis, and in patients who present with an embolic stroke. However, there is no consensus on the optimal management of asymptomatic patients with CCMA. Based on our review, it would be reasonable to consider elective surgical resection of CCMA masses in asymptomatic patients who are good surgical candidates, and also in selected elderly patients, because anticoagulation and antiplatelet therapy are ineffective to prevent a cardioembolic stroke in patients with CCMA.
STUDY LIMITATIONS
One possible source of bias is that patients who present with adverse events (like a stroke) are more likely to be published as case reports, and the exact denominator of patients with CCMA (without a stroke) is not known. Also, the precise prevalence of AF in the published CCMA cases is unknown, because the articles reviewed on patients with CCMA presenting with a stroke did not indicate whether continuous electrocardiographic monitoring or Holter were used to enhance detection of AF, except for one paper [28]. Unfortunately, the only way to determine the incidence of stroke in patients with CCMA would be to follow for several years a cohort of patients with and without CCMA who were stroke-free at baseline, and determine whether those with CCMA have a higher risk of subsequent strokes, independent of other risk factors. However, this type of study would be impractical because of the small number of patients with CCMA.
ETHICS REVIEW
The institutional Human Research Review Committee has reviewed and approved this research study on January 16, 2014. A copy of the New Study Approval is available for review by the Editor-in-Chief of this journal on request.
APPENDIX
All cases of CCMA reported in the English-language literature, listed in chronological order of publication.
| Author | Year | Pts (n) | Age | Sex | Symptoms | Risk factors | TTE findings | Treatment | F/U |
|---|---|---|---|---|---|---|---|---|---|
| Pomerance [14] | 1970 | 7 | 50-90 | 6F | ? | ? | ----- | ----- | ----- |
| 1M | ? | ? | ----- | ----- | ----- | ||||
| Kronzon | 1983 | 3 | 73 | F | syncope | HT | calcified mass | observation | sudden death |
| 23 | M | CHF | ? | calcified mass | pericardiectomy | ----- | |||
| 61 | M | CHF | CAD + AF | calcified mass + severe MR | resection + MVR | ----- | |||
| Teja | 1987 | 1 | 57 | F | DOE | HT | cardiac tumor | resection of tumor | ----- |
| Namura [33] | 1992 | 1 | 60 | F | asymptomatic | ESRD + HT | calcified mass | observation | death (CVE) |
| Gilbert | 1997 | 1 | 65 | F | DOE | ? | MV abscess + severe MR | resection + MVR | ----- |
| Isotalo | 1999 | 1 | 42 | M | empyema | ESRD+HT+CAD | ----- | observation | death |
| Koito | 1999 | 1 | 58 | F | asymptomatic | ESRD | EM/CL | observation | regression |
| Harpaz [10] | 2001 | 19 | 54-76 | 14F | CVE 5 + sync 2 | HT 9 + DM 4 | EM/CL 19 | observation 16 | 3 deaths |
| 5M | CHF 1+DOE 8+palpit 1 | ESRD 1 + AF 2 | severe MR 1 + severe MS 1 | MVR 3 | 1 death | ||||
| Morgan | 2003 | 1 | 83 | F | ? | ? | EM/CL | observation | ----- |
| Novaro | 2004 | 1 | 74 | F | lethargy | ? | EM/CL | observation | ----- |
| Alkadhi | 2005 | 1 | 70 | F | DOE + syncope | HT | EM/CL + severe MR | resection + annuloplasty | ----- |
| De Conti | 2005 | 2 | 64 | F | DOE | HT | calcified mass | observation | ----- |
| 63 | F | DOE | HT + DM | calcified mass | observation | ----- | |||
| Gramenzi | 2005 | 1 | 60 | F | asymptomatic | ESRD + HT | EM/CL | observation | resolution |
| Puri | 2005 | 1 | 57 | F | angina | CAD | MAC + dyn LVOTO | resection + MVR | ----- |
| Davidson [15] | 2006 | 1 | 79 | F | CVE (retina) | HT | calcified mass + severe MR | resection + MVR | ----- |
| de Vrey | 2006 | 1 | 61 | M | DOE | HT+ DM+ DLP | calcified mass in LV | resection + annuloplasty | ----- |
| Di Bella | 2006 | 1 | 84 | F | DOE | HT + AF | calcified mass + moderate MR | observation | ----- |
| Izgi | 2006 | 1 | 62 | F | angina | HT + DLP | EM/CL | observation | ----- |
| Kato | 2006 | 1 | 56 | F | DOE | ESRD + AF | cardiac tumor + severe MS | resection + MVR | ----- |
| Marcu | 2006 | 1 | 70 | M | asymptomatic | CAD (CAB) | calcified mass | observation | ----- |
| Vanovermeire | 2006 | 1 | 79 | F | DOE | HT + DLP | myxoma + severe MR | resection + MVR | death |
| Fernandes [12] | 2007 | 6 | 69 | F | DOE + palpitations | HT + AF | EM/CL + moderate MR | observation | ----- |
| 79 | F | DOE | HT | EM/CL + severe MR | refused surgery | ----- | |||
| 79 | F | palpitations | HT + DLP | EM/CL | observation | ----- | |||
| 76 | F | CHF | HT + AF | EM/CL + severe MR | resection + MVR | ----- | |||
| 65 | M | CHF | HT + DLP | calcified mass + severe MR | resection + annuloplasty | ----- | |||
| 81 | F | MI + CHF | HT + DM | calcified mass + severe MR | resection + MVR | ----- | |||
| Johanssen | 2007 | 1 | 58 | M | possible IE | ESRD + HT | possible vegetation | observation | ----- |
| Lubarsky [16] | 2007 | 1 | 70 | F | CVE | HT+ DM (NSR) | MAC | observation | ----- |
| Poh [17] | 2007 | 1 | 74 | F | CVE | HOCM | cardiac tumor | resection of tumor | ----- |
| Yokoyama | 2007 | 1 | 54 | M | asymptomatic | ESRD + AVR | EM/CL | observation | progression |
| Zeina | 2007 | 1 | 55 | M | DOE | HT + DLP | calcified mass | observation | regression |
| Arora | 2008 | 1 | 71 | M | syncope | ? | EM/CL | observation | ----- |
| Deluca [11] | 2008 | 14 | 59-79 | 10F | syncope 2 + MI 1 | ESRD 2+ AF 5 | EM/CL 14 + moderate MR 1 | observation | CVE 1 + sync 2 |
| 4M | CHF 1 + DOE 2 | HT 14 + DM 5 | observation | regression 3 | |||||
| palpitations 6 | DLP 6 | observation | progression 3 | ||||||
| Di Bella | 2008 | 1 | 68 | M | angina | ? | calcified mass | resection + MVR+CAB | ----- |
| Minardi | 2008 | 1 | 69 | F | CHF | DLP + PAF | EM/CL + severe MR | resection + annuloplasty | ----- |
| Monti | 2008 | 2 | 87 | F | asymptomatic | ? | cardiac tumor | observation | ----- |
| 70 | M | asymptomatic | ? | cardiac tumor | observation | ----- | |||
| Biteker | 2009 | 1 | 73 | F | DOE + palpitations | HT | calcified mass | observation | ----- |
| Blankstein | 2009 | 1 | 66 | M | ? | ? | calcified mass | observation | progression |
| Correale | 2009 | 1 | 76 | F | asymptomatic | HT | MV tumor | observation | resolution |
| Durăo | 2009 | 1 | 73 | F | MI (embolus) | ? | EM/CL | resection + MVR | ----- |
| Fernàndez | 2009 | 1 | 75 | F | asymptomatic | HT + PAF | MV tumor | observation | ----- |
| Marci | 2009 | 2 | 82 | F | palpitations | HT + PAF | EM/CL | observation | ----- |
| 71 | F | MI | ? | EM/CL | observation | ----- | |||
| Salisbury | 2009 | 1 | 62 | M | DOE | AF | EM/CL + severe MR | refused surgery | ----- |
| Vizzardi | 2009 | 1 | 65 | F | DOE | HT + DLP | EM/CL | observation | ----- |
| Warsame | 2009 | 1 | 60 | M | angina | HT+DLP+CAD | EM/CL | observation | ----- |
| Assudani | 2010 | 1 | 77 | F | syncope | HT+HOCM | EM/CL+severe MR+dyn LVOTO | MVR + myectomy | ----- |
| Higashi [18] | 2010 | 1 | 76 | F | CVE | ESRD+HT(NSR) EM/CL | anticoagulation | ----- | |
| Jiménez | 2010 | 1 | 63 | M | DOE | ? | calcified mass | observation | ----- |
| Rojas | 2010 | 1 | 67 | F | angina + VTach | ? | tumor + moderate MR | ? | ? |
| Shriki | 2010 | 3 | 63 | M | asymptomatic | (colon cancer) | ----- | observation | ----- |
| 84 | F | DOE | HT+DM+DLP | calcified mass | observation | ----- | |||
| 82 | M | DOE | ? | possible myxoma | observation | ----- | |||
| Spina | 2010 | 1 | 81 | M | DOE | ? | calcified mass + severe MR | resection + MVR | ----- |
| Stamou [19] | 2010 | 1 | 84 | M | CVE (retina) | ? | tumor + severe MR+AS | MVR + AVR | ----- |
| Chahal | 2011 | 1 | 73 | F | angina | ? | EM/CL + dyn LVOTO | observation | ----- |
| Chevalier [20] | 2011 | 1 | 72 | F | CVE | HT | calcified cyst | resection + annuloplasty | ----- |
| Collins | 2011 | - | - | - | ----- | ----- | ----- | ----- | ----- |
| Dubrey | 2011 | 2 | 78 | F | DOE | HT + AF | cardiac tumor + AS | observation | ----- |
| 81 | F | CHF | HT +AF | tumor + moderate MR | observation | ----- | |||
| Garcia-I. | 2011 | 3 | 66 | F | angina | HT + DLP | MV tumor | resection + CAB | ----- |
| 72 | F | syncope | HT + CHB | calcified tumor | pacemaker | ----- | |||
| 71 | F | syncope | BFB | EM/CL | ? | ? | |||
| Gulati [21] | 2011 | 1 | 77 | M | CVE (TIA) + DOE | HT + DLP | EM/CL + severe AS | refused surgery | ----- |
| Pozsonyi | 2011 | 1 | 65 | F | CHF | AF | calcified mass + severe MR | resection + MVR | ----- |
| Providencia | 2011 | 1 | 74 | F | angina | HT + CAD | cardiac tumor + moderate MR | observation | ----- |
| Vijayan [22] | 2011 | 1 | 81 | F | CVE | HT+HLD (NSR) | calcified mass | anticoagulation | ----- |
| Akpinar | 2012 | 1 | 68 | F | syncope | ESRD + HT | EM/CL + dyn LVOTO | resection + MVR | ----- |
| Akram [23] | 2012 | 1 | 59 | F | CVE | ESRD + HT | EM/CL | observation | ----- |
| Chen [24] | 2012 | 1 | 76 | F | CVE + angina | HT + DLP | calcified mass | observation | progression |
| Fujiwara | 2012 | 1 | 73 | F | syncope | CHB | cardiac tumor + moderate MS | resection + MVR | ----- |
| Lee | 2012 | 1 | 60 | M | angina | HT + DM | cardiac tumor | resection + CAB | ----- |
| Martínez | 2012 | 1 | 76 | F | palpitations | AF + DLP | EM/CL | observation | ----- |
| Matsuyama [25] | 2012 | 1 | 92 | F | CVE + CHF | HT+DM+DLP | MAC | observation | death (CVE) |
| McKernan [26] | 2012 | 1 | 84 | F | CVE + MI | ? | myxoma + moderate MR | resection + annuloplasty | death (reop) |
| Motwani [27] | 2012 | 1 | 64 | M | CVE | ? | MAC | observation | regression |
| Ribeiro | 2012 | 1 | 84 | F | CHF | HT + DLP | calcified mass + severe MR | refused surgery | ----- |
| Srivatsa | 2012 | 1 | 75 | M | asymptomatic | HT+DLP+CAD | calcified mass | observation | ----- |
| Ueyama | 2012 | 1 | 76 | F | ? | ? | cardiac tumor | resection + MVR | ----- |
| Alizade | 2013 | 1 | 79 | F | CHF | ? | cardiac tumor + severe MR | surgery (?) | ? |
| Alzuhairi | 2013 | 1 | 67 | F | fatigue | HT+DM+DLP | possible myxoma | observation | ----- |
| Corre [28] | 2013 | 1 | 83 | F | CVE | (NSR) | tumor + moderate MR | anticoagulation | ----- |
| Elgendy [13] | 2013 | 1 | 41 | F | DOE + IE | ESRD + DM | EM/CL + severe MS | resection + MVR | ----- |
| França | 2013 | 1 | 83 | F | DOE | HT + DLP | EM/CL | observation | ----- |
| Kalayci [29] | 2013 | 1 | 59 | F | CVE | HT (NSR) | calcified mass + severe MR | resection + MVR | ----- |
| Kuwauchi | 2013 | 1 | 69 | M | angina | HT+DM+DLP | ----- | CAB only | ----- |
| Kydd | 2013 | 1 | 77 | M | syncope | ? | cardiac tumor | ? | ? |
| Mallat | 2013 | 1 | 82 | F | asymptomatic | HT + DLP | calcified mass | observation | ----- |
| Plank | 2013 | 1 | 61 | F | asymptomatic | HT | calcified mass + LVOTO | observation | ----- |
| Pomeroy | 2013 | 1 | 90 | M | angina | DM + CAD | LA tumor vs MAC | observation | ----- |
| Pugliatti [30] | 2013 | 1 | 78 | F | CVE | DM (NSR) | LA thrombus + moderate MR | observation | ----- |
| Demirkol | 2014 | 1 | 78 | F | DOE | HT+DM+DLP | calcified mass | observation | ----- |
| Mallisho [31] | 2014 | 1 | 78 | F | CVE | (NSR) | EM/CL | refused surgery | ----- |
| Możeńska | 2014 | 1 | 76 | F | asymptomatic | HT+DM+DLP | possible myxoma | observation | ----- |
| Sequeira [32] | 2014 | 1 | 39 | F | CVE + CHF | ESRD + HT | MAC + severe MS | resection + MVR | ----- |
| Shah | 2014 | 1 | 77 | F | DOE | ? | LA tumor + moderate MR | observation | ----- |
AF = atrial fibrillation; AS = aortic stenosis; AVR = aortic valve replacement; BFB = bifascicular block; CAB = coronary artery bypass; CAD = coronary artery disease; CCMA = caseous calcification of the mitral annulus; CHB = complete heart block; CHF = congestive heart failure; CVE = cerebrovascular event; DLP = dyslipidemia; DM = diabetes mellitus; DOE = dyspnea on exertion; dyn = dynamic; EM/CL = echogenic mass with central lucencies; ESRD = end-stage renal disease; F = female; F/U = follow-up; HOCM = hypertrophic obstructive cardiomyopathy; HT = hypertension; IE = infective endocarditis; LA = left atrium; LVOTO = left ventricular outflow tract obstruction; M = male; MAC = mitral annulus calcification; MI = myocardial infarction; MR = mitral regurgitation; MS = mitral stenosis; MV = mitral valve; MVR = mitral valve replacement; NSR = normal sinus rhythm; PAF = paroxysmal atrial fibrillation; palpit = palpitations; reop = reoperation; sync = syncope; TEE = transesophageal echo; TIA = transient ischemic attack; TTE = transthoracic echo; VTach = ventricular tachycardia.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
The authors are very grateful to Michael G Lopez, CCT (Cardiology PACS Administrator) for his technical assistance with echocardiography imaging.


