All published articles of this journal are available on ScienceDirect.
Comparison of the Effects of Dialysis Methods (Haemodialysis vs Peritoneal Dialysis) on Diastolic Left Ventricular Function Dialysis Methods and Diastolic Function
Abstract
Introduction:
In patients undergoing chronic dialysis, several factors appear to influence the occurrence of cardiac abnormalities. The aim of our study was to evaluate the effects of two different methods of renal replacement therapy (chronic haemodialysis (HD) and continuous ambulatory peritoneal dialysis (CAPD)) on left ventricular (LV) diastolic function.
Patients and Methods:
We enrolled 63 patients: 21 patients on CAPD, and 42 age- and gender-matched patients on HD; 35 patients were men (55.6%). Median of age was 46.4 (35-57) years. The median duration of renal replacement therapy was 3(2-5) years.
Results:
The two groups (HD vs PD) were similar concerning body mass index, dialysis duration and cardiovascular risk factors. The comparison of echocardiographic parameters showed statistically significant differences between two groups, regarding the presence of calcification, cardiac effusion, severely abnormal left ventricular hypertrophy(LVH) and the ratio of mitral velocity to early diastolic velocity of the mitral annulus (E/e’) >13 (p= 0.001, p= 0.003, p= 0.02, p= 0.004, respectively). In multivariate analysis, an E/e’>13 was higher in PD group ( OR= 5.8, CI [1.3-25.5], p=0.002).
Conclusion:
The method of dialysis seems to influence LV diastolic function. We observed a higher prevalence of diastolic LV dysfunction in the PD group. Echocardiographic follow up is essential as this could improve the management of cardiovascular complications in dialysis patients.
INTRODUCTION
Echocardiography is the most useful imaging technique for initial cardiac assessment, enabling detailed examination of the main cardiac structures and effective assessment of the left ventricular (LV) mass and changes in ventricular function [1]. Indeed, in patients undergoing chronic dialysis, left ventricular hypertrophy (LVH) and cardiac geometry influence LV dysfunction [2]. Several factors appear to influence the occurrence of cardiac abnormalities. Whether haemodialysis (HD) or peritoneal dialysis (PD) has a different impact on echocardiographic parameters has been previously investigated, but the results are heterogeneous and contradictory.
The aim of our study was to evaluate the effects of two different methods of renal replacement therapy (chronic HD and continuous ambulatory peritoneal dialysis (CAPD)) on echocardiographic parameters.
PATIENTS AND METHODS
Patients
We enrolled 63 patients; 21 patients on CAPD, and 42 age- and gender-matched patients on HD after obtaining informed consent.
35 patients were men (55.6 %). The median of age was 46.4 (35-57) years. The median of duration of renal replacement therapy was 3 (2-5) years.
Haemodialysis was performed three times a week for 4 h. Echocardiographic parameters were measured within 2 h after a dialysis session or peritoneal exchange.
Comparison of the use of antihypertensive drugs in the two groups of the study.
Inclusion Criteria
We included patients on renal therapy replacement for >6 months, with an adequate acoustic window for the echocardiography.
Exclusion Criteria
We excluded patients with severe anaemia, uncontrolled hypertension, diabetes, rhythm or conduction abnormality, valvular heart disease, past history of heart failure or unstable angina.
Methods
Therapeutic Modalities
CAPD consists of 3 to 4 exchanges/day. All haemodialysis patients had a radial arteriovenous fistula. Haemodialysis was carried out three times a week for 4 h with standard bicarbonate dialysis.
Clinical Data
Baseline characteristics were collected: age, gender, dialysis duration (in years), hypertension, hyperlipidaemia and smoking.
Hypertension was defined as systolic blood pressure (BP) ≥140 mmHg, diastolic BP ≥90 mmHg or the use of antihypertensive medication. For hypertensive patients, the strict control of BP was required and treatment with renin-angiotensin-system inhibitors was introduced.
Hyperlipidaemia was defined as total cholesterol ≥200 mg/dL, low-density lipoprotein cholesterol (LDL-C) ≥130 mg/dL or the use of lipid-lowering medication.
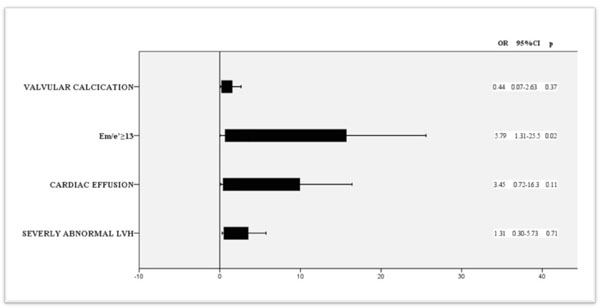
The association between dialysis methods and echocardiographic parameters. Multiple logistic regression model of statistically significant regressors.
Biological Data
Routine laboratory methods were used to measure biochemical parameters: haemoglobin, C-reactive protein (CRP), phosphorus, calcium, rate of intact parathyroid hormone (iPTH). Residual renal function (RRF) was estimated by calculating glomerular filtration rate (GFR). GFR was calculated ref according to the formula: GFR = Uvol/U × {Uurea[(PreUrea + PostUrea)/2] + Ucreat/[(PreCreat +PostCreat)/2]} calcuSA (SA: surface area in m2, t: duration of collection between dialyses in minutesUvol: urine collection volume in mL, PreUrea and PreCreat: pre-dialysis urea and creatinine concentration in blood samples at the end of the collection, PostUrea and PostCreat: post-dialysis urea and creatinine concentration in blood samples at the beginning of collection, and : urea and creatinine urine concentrations) [3]. The surface area (SA) was estimated by the Mosteller formula: SA= √L x M/3600 (SA in m2, weight in Kg, height in cm) [4].
Clinical and laboratory criteria for adequate nutrition were an albumin ≥ 35 g/l and a body mass index (BMI) ≥ 21 kg/m2. Anaemia was defined as haemoglobin<11g/dl.
Echocardiographic Data
Echocardiography was achieved by a General Electric, Vivid 7 ultrasound system. The same cardiologist performed all echocardiographic examinations. The patients were examined in the left lateral decubitus position and data was collected in standard parasternal long-axis, short-axis, and apical views. Measurements were acquired by averaging three cardiac cycles.
Left ventricular hypertrophy is defined by an increase in LV mass (LVM) index > 95 g/m2 in women and > 115 g/m2 in men. LVM was considered as severely abnormal if LVM ≥122 g/m2 in women and ≥149g/m2 in men.
The following anatomically validated equation was used to estimate LVM: LVM = 0.8[1.04(IVS + LVIDd + LVPW)3 – LVIDd3] + 0.6; where IVS is the diastolic septal thickness, LVIDd is the diastolic dimension of the LV cavity and LVPW is the diastolic thickness of the posterior wall [5].
Teicholz’s and Simpson’s methods were used to evaluate LV ejection fraction. Echocardiographic maximum left atrial volume was measured from apical 4- and 2-chamber views by biplane area-length [6, 7].
The values of the E wave and A wave of mitral flow were collected (on apical 4-chamber view, a 3 mm, at the level of the basal mitral annulus). Tissue Doppler velocities of longitudinal mitral annular motion were measured in median and lateral segment of the basal mitral annulus (on apical 4-chamber view) and the mean e’ (early diastolic velocity) was used to estimate E/e’.
Peak systolic velocity (S), early (e') diastolic velocities were assessed for right and left annular side, successively.
Statistical Data
Continuous variables are expressed as mean ± standard deviation (or median with inter-quartile range (IQR) when the distribution is non-normal). Qualitative variables were analysed using the Chi2 test (or the Fisher exact test according to the number of cases), and quantitative variables were analysed using the t test (or a nonparametric test according to the normality of distribution). Statistical significance was defined as P < 0.05. Statistical analysis was performed using SPSS for Windows, version 11.0.
Comparison of demographic and biological characteristics.
| HD group n=42 |
PD group n=21 |
p | |
|---|---|---|---|
| Male gender (%) Age (years) |
21 (50) 44.8[33.7-52] |
14 (66.6) 51[39-57.5] |
0.45 0.13 |
| BMI (Kg/m²) | 22.8±3.7 | 22.6±6.8 | 0.57 |
| Weight (kg) Hypertension (%) |
65.2±14.2 18(45) |
67.5±14.5 13(61.9) |
0.39 0.21 |
| Dialyse duration (years) | 3[2-6] | 3[2-4.5] | 0.07 |
| Triglycerides (mmol/l) | 1.1 [0.8-1.5] | 1.3 [0.9-1.8] | 0.45 |
| LDL-C (mmol/l) CRP (mg/l) |
1.1 [0.9-1.27] 4[2.3-6] |
1.1 [0.9-1.4] 4[2-6] |
0.7 0.6 |
| Uric acid (mg/l) Calcium (mg/dl) Phosphorus (mg/dl) Anaemia (%) Smoking (%) |
61[55-76] 9 ±0.8 47[28-65] 4(9.5) 5(12) |
72[60-80] 8.6 ±0.9 49.8[44-61.5] 3(14.2) 4(19) |
0.08 0.12 0.051 0.7 0.7 |
RESULTS
The mean body weight was 66 ±14.2 kg. Hypertension was noted in 50.8 % (31 cases). Antihypertensive drugs were similarly used in the two groups (Fig. 1). Nine patients (14.8%) were smokers, and 21 patients (33.3 %) had dyslipidaemia. The two groups (HD vs PD) were similar concerning body mass index, dialysis duration, residual renal function, anaemia and cardiovascular risk factors. Comparison of biological characteristics showed no statistically significant difference between the two groups. Data is reported in Table 1. In our study, LVH was noted in 28 patients (44.4%) and LVM was considered as severely abnormal in 19 patients (20.2%). There was no statistically significant difference between the two groups concerning LVH (p=0.45) but severely abnormal LVH was more frequent in PD group (23.8% in HD group vs 42.9% in PD group, p=0.02). There was a significant difference between the two groups concerning the presence of calcification, the presence of cardiac effusion and E/e’ >13 (p= 0.001, p= 0.003, p= 0.004, respectively). The comparison of echocardiographic parameters is shown in Tables 2 and 3. Multivariate logistic regression was used to evaluate the association of each significant echocardiographic parameters with dialysis methods. All significant variables were considered candidate confounders. In this regression model, and after adjustment for confounders, an E/e’>13 was higer in PD group CI(OR= 5.8;CI [1.3-25.5], p=0.002) (Fig. 2).
DISCUSSION
Is it Relevant to Measure Diastolic Function of the LV?
Mortality is known to increase with LV diastolic dysfunction [8].
Hsiao and al showed that in the uraemic population, LV dysfunction, LVM index and a high post-dialytic E/e’ are prognostic of major events [8]. In the same way, in a study by Kim and al, diastolic dysfunction was a significant marker of rapid decline in residual renal function and the occurrence of cardiovascular events in patients placed in a peritoneal dialysis program [9].
What are the Methods of Evaluation of Diastolic Function?
In CKD (chronic kidney disease) patients, TDI is more sensitive to detect diastolic dysfunction than conventional echocardiography [10-13]. Despite the relative fluid deficiency after HD, which can induce a decrease of Em and Am, it is very important to evaluate all parameters of LV diastolic function in this population [7]. Apart from LVM and systolic function, the E/Em ratio displayed important additional long-term prognostic information [14].
Comparison of Comparison of the two-dimensional and M-mode findings.
| HD Group n= 42 |
PD Group n= 21 |
p | |
|---|---|---|---|
| LVEDD (mm) LVEDD index (mm/m2) LVESD (mm) LVESD index (mm/m2) IVS (mm) PW (mm) RWT LVM (g) LVM index (g/m2) LVH (n (%)) Severely abnormal LVH (n(%)) EF (%) RVEDD (mm) TAPSE (mm) RVS (mm) |
51.2±5.6 30.1±4 32.1±8 19.2±4.7 9.6±2.6 9.7±2.3 0.38±0.09 174[131-220] 124[88-170] 17 (40.5%) 10 (23.8%) 64.3±9.7 24±4.4 23.3±3.4 14.3±2.2 |
51.2±8.9 30±4.9 30.2±4.9 18.2±2.8 10.6±2.6 10.3±2 0.41±0.1 210[150-242] 108[76-141] 11(52.4%) 9(42.9%) 64.7±12.3 22.2±3.9 24.4±3.8 15±1.4 |
0.41 0.33 0.18 0.11 0.07 0.2 0.18 0.19 0.14 0.45 0.02 0.38 0.13 0.52 0.36 |
| IVC diameter (mm) RA Area (cm2) LA Area (cm2) LA volume (ml) PASP (mmHg) Valvular calcification (n(%)) |
9[6-11] 16.7±7.9 17.8±6.6 44[33-77.5] 30.8±9.9 9(21.4%) |
10.6[7.5-13.5] 13.8±2.7 18.4±3.9 57 [39-72] 31±7.8 2(9.5%) |
0.05 0.77 0.44 0.78 0.81 0.001 |
Comparison of filling pressures features between the two groups.
| HD Group n= 42 |
PD Group n= 21 |
p | |
|---|---|---|---|
| Em/Am ≥2 | 0(0%) | 1(4.7%) | 0. 09 |
| Em/e’ ≥13 | 5(11.9%) | 8(38.1%) | 0.004 |
| PASP >35mmHg | 5(11.9%) | 1(4.7%) | 0.55 |
| Pericardial effusion | 5(11.9%) | 10(47.6%) | 0.003 |
| LA volume >52ml | 15(35.7%) | 7(33.3%) | 0.36 |
What are the Effects of Dialysis Methods on LV Diastolic Function?
Diastolic dysfunction has been observed in patients receiving renal replacement therapy for ESRD in many studies [15, 16]. In a study by Duran et al., diastolic function of LV was not significantly altered after maintenance haemodialysis treatment. They demonstrated that in the long run, the acute changes of volume, electrolytes and autonomic regulation due to haemodialysis session does not affected left diastolic function [17]. For Lee et al., the prevalence and severity of diastolic LV dysfunction is higher in PD patients [18].
Some authors suggest that LVM and diastolic function are closely related to each other in all dialysis patients [19]. Moreover, in a study by Kimura and al., LVMI was an independent determinant of LV diastolic dysfunction in patients on HD [15]. The prevalence of LVH was higher in conventional HD compared to PD [15]. According to the Framingham criteria, its prevalence was 68.8% in HD patients and 45.2% in PD patients [20]. According to that observation, we introduced LVH in our consideration in the evaluation of difference between the two groups concerning LV diastolic function. In fact, in the present study, we observed that the prevalence of LVH was 44.4% (40.5% in HD patients and 52.4% in PD patients, p= NS). This difference with literature data can be explained by higher age and higher frequency of hypertension in our PD group (although the comparative analysis finds no significant difference concerning those parameters).
However, we introduced severe LVH in a multivariate regression model. We noticed a higher prevalence of diastolic LV dysfunction in the PD group. We also noted that an E/e’ >13 is the stronger parameter for detection of this LV diastolic dysfunction.
LIMITATION OF OUR STUDY
Invasive haemodynamics to explore more filling pressure data were not included. The strain and CMR were not performed, that is why we have not been able to explore further the systolic function of the right ventricle.
ETHICAL APPROVAL
This study was carried out retrospectively; therefore, no ethics committee approval was required. There is no patient identification within this publication.
LIST OF ABBREVIATIONS
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.


