All published articles of this journal are available on ScienceDirect.
The Effects of Levosimendan and Sodium Nitroprusside Combination on Left Ventricular Functions After Surgical Ventricular Reconstruction in Coronary Artery Bypass Grafting Patients
Abstract
Objective:
The aim of our study was to research the effects of levosimendan (LS) and sodium nitroprusside (SNP) combination on systolic and diastolic ventricular function after coronary artery bypass grafting (CABG) who required endoventricular patch repair (EVPR).
Patients and Methods:
We studied 70 patients with ischemic dilated cardiomyopathy. LS and SNP combination was administered in 35 patients (study group, SG). In the remaining patients, normal saline solution was given (placebo group, PG). Levosimendan (10µgr/kg) started 4 h prior to operation and we stopped LS before the initiation of extracorporeal circulation (ECC). During the rewarming period, we started again levosimendan (10µgr/kg) in combination with SNP (0.1-0.2 µgr/kg/min). If mean blood pressure decreased by more than 25% compared with pre-infusion values, for corrected of mean arterial pressure, the volume loading was performed using a 500 ml ringer lactate. Hemodynamic variables, inotrophyc requirement, and laboratory values were recorded.
Results:
Five patients died (7.14%) post-surgery (one from SG and 4 from PG) due to low cardiac out-put syndrome (LOS). At the postoperative period, cardiac output and stroke volume index was higher in SG (mean±sd;29.1±6.3 vs. 18.4±4.9 mL/min−1/m−2 (P<0.0001)). Stroke volume index (SVI) decreased from 29±10mL/m2 preoperatively to 22±14mL/m2 in the early postoperative period in group 1. This difference was statistically significant (P=0.002). Cardiac index was higher in SG (320.7±37.5 vs. 283.0±83.9 mL/min−1/m−2 (P=0.009)). The postoperative inotrophyc requirement was less in SG (5.6±2.7 vs. 10.4±2.0 mg/kg, P< 0.008), and postoperative cardiac enzyme levels were less in SG (P< 0.01). Ten patients (28.5%) in SG and 21 patients (60%) in PG required inotrophyc support (P<0.001). We used IABP in eight patients (22.8%) in SG and 17 patients (48.5%) in CG (P=0.0001).
Conclusion:
This study showed that LS and SNP combination impressive increase in left ventricular systolic and diastolic functions including LVEF. The use of this combination achieved more less inotrophics and IABP requirement. We therefore suggest preoperative and peroperative levosimendan and SNP combination.
INTRODUCTION
Left ventricular aneurysm and cardiomyopathy remains a serious disorder that can lead to intractable congestiveheart failure (CHF), anddeath. The goal of surgical interventionis to correct the size and geometry of the LV, reduce wall tension, and improve ventricular systolic function [1]. EVPR was desribed as a more physiologicrepair than the linear closure technique by Dor and Cooley [2, 3]. Mickleborough and co-workers have introduced a method combining septoplasty and linear resection [4]. Pharmacologic therapies for reducing myocardial afterload and preload has been recommended to induce myocellular and molecular reverse remodeling after surgery [5-7]. However, EVPR may be cause of detrimentally affect on LV diastolic properties [8]. Therefore, pharmacologic treatment options are very important after surgery. Levosimendan, as a calcium sensitizer, is used in cardiac surgery to provided the myocardial preconditioning and vasodilating effects without the intracellular calcium accumulation during the ischemia-reperfusion injury. Also, previous investigations have shown that SNP infusion reduces ventricular pre- and afterload after myocardial ischemia by favorably influencing the balance between myocardial oxygen supply and demand [9, 10]. SNP has also suggested for left ventricular relaxation by causing reflex changes in the autonomic nervous system and stimulating catecholamine release. Previous investigation by Freyholdt et al. showed that a SNP infusion improved the cardiac index immediately after reperfusion and at the end of surgery [9]. SNP also reduced the cardiac inflammatory response during CABG in patients with severely compromised left ventricular function [10]. According to previous data, SNP may be capable of influencing the myocardial relaxation process under hypoxic conditions and may alter viscoelastic properties of the left ventricle after SVR. To provide dentrimental effects of EVPR we hypothesised that pharmacologic preconditioning may be help to increase left ventricular systolic amd diastolic functions. To research of the combined drugs effects on left ventricular functions we used LS and SNP for preoperative pharmacologic preconditioning.
MATERIALS AND METHODS
After the approve of ethics committee, we studied 70 CABG patients with LVA who required EVPR between January 2007 and July 2014. All patients have had previous one or more myocardial infarction. The preoperative patients’ charecteristics are summarized in Table 1. Criteria for exclusion included a planned concomitant valve procedure, emergency surgery, history of persistent ventricular tachycardia or obstructive cardiomyopathy, myocardial infarction within 72 h before surgery, preoperative inotropic support, or use of an intraaortic balloon pump at the time of surgery. Indications for EVPR were heart failure, angina or a combination of these symptoms. Stroke volume was defined as the difference between end-diastolic volume (EDV) and end-systolic volume (ESV). LV volume measurements were indexed to body surface area and expressed as end-diastolic volume index (EDVI), end-systolic volume index (ESVI), and stroke volume index (SVI). Preoperatively, angiocardiography including left ventricular end-diastolic pressure was calculated.
Baseline characteristics of the study groups.
| Characteristic | LS+SNP | CG | P Value |
|---|---|---|---|
| (N=50) | (N=46) | ||
| Age (years) | 59.4±7.1 | 56.4±5.4 | 0.095 |
| Sex (males/females) | 40/10 | 35/11 | 0.228 |
| Smoking | 21 | 20 | 0.349 |
| Obesity | 15 | 10 | 0.406 |
| Hypertension | 14 | 6 | 0.047 |
| Impaired renal function* | 4 | 6 | 0.94 |
| Diabetes mellitus | 8 | 7 | 0.90 |
| Mean interval between MI and the operation (years) | 3 | 2.8 | 0.95 |
| NYHA class II–III | 46 | 43 | 0.88 |
| Angina pectoris | 29 | 27 | 0.94 |
| Stable | 17 | 15 | – |
| Unstable | 9 | 7 | – |
| Thromboembolism | 0 | 0 | 1.0 |
| Mitral regurgitation (grade1–2) | 3 | 2 | 0.94 |
| CAD | |||
| 2-vessel | 16 | 14 | 0.94 |
| 3-vessel | 34 | 32 | 0.92 |
| Mean LVEF | 36.7±5.4 | 38.4±7.2 | 0.658 |
| Mean LVEDP (mmHg) | 16.5±9.7 | 13.1±5.8 | 0.546 |
In SG, levosimendan (10µg/kg) started 4 h prior to operation. We continued levosimendan administration through central venous catheter until ECC. During the rewarming period, we started levosimendan infusion again (10µgr/kg in combination with SNP (0.1-0.2 µgr/kg/min.).). If mean blood pressure decreased by more than 25% compared with pre-infusion values, the volume loading was performed using a 500 ml ringer lactate for correction of arterial pressure. CG received placebo infusion (0.9% of normal saline) in accordence with LS and SNP infusion protocol. In both groups, echocardiographyc examination was done before the discharge from hospital.
Surgical Procedure
After midline sternal insicion, aortic and caval cannulation was performed and CPB was instituted. Cardiac arrest has been provided antegrade and retrograde cold blood cardioplegic solution every 20 min. An aneurysmal suc has been opened and resected in all patients. If there was a thromby, we removed by carefully attention. A pursestring suture was placed around the circumferential scar tissue at the transition zone and a circular Dacron or pericardial patch was secured over the opening after a pursestring suture was tied. If there was a significant septal lesion in addition to the anterior-apex lesions, we used a septal anterior ventricular exclusion procedure after a long left ventriculotomy was made along the left anterior descending artery, an elliptical-shaped Dacron patch was sutured to the transition zone to exclude the akinetic region after broad anteroseptal infarction. Coronary bypass anastomosis were performed using a saphenous vein and a left internal thoracic artery (LITA). During rewarming period, in SG, SNP and levosimendan was administered via central venous line at a dose of 0.1-0.2 mg/kg/min., and 5-10µg/kg, respectively. In PG, normal saline solution was given. Proxymal anastomosis were completed using a side clamp. We continued Levosimendan and SNP in ICU for 24 h. The characteristics of surgical procedures are summarized in Table 2. For all patients angiotensin-converting enzyme inhibitor, β-blocker, and diuretics were used. Medication and basic features of the surgical procedures were comparable. There were no differences in heart rate that might have influenced SVI changes after SVR.
Operative data.
| Parameters | LS+SNP(N=50) | CG(N=46) | P value |
|---|---|---|---|
| Mean CPB time (min) | 85.1±23.9 | 94.9±26.2 | 0.083 |
| Mean aortic cross-clamp time (min) | 52.3±14.9 | 49.7±11.9 | 0.076 |
| Concomitant CABG | 50 | 46 | 1.0 |
| Mean number of grafts (grafts per patient) | 2.74 | 3.01 | 0.670 |
| Urgent operations | 8 | 5 | 0.802 |
Statistical Analysis
Continuous variables are expressed as mean ± standard deviation. Categorical variables are presented as percentages. The Student t test was used to compare preoperative and postoperative continuous variables, the chi-squared test and Fischer’s exact test were used for analysis of categorical variables. Univariate regression analysis was used to determine factors associated with early hospital mortality and a low cardiac output state. Survival curves were calculated according to the method of Kaplan - Meier and subgroups were compared using the Log-rank test. Soft ware version 13.0 for Windows was used in data analysis. A significant difference was considered at P<0.05.
RESULTS
Five patients died after operations due to LOS (Four in PG (11.4%), and one in SG (2.8%). This was statistically significant (P<0.001). The mortality risk factors were duration of ECC (exceeding 220 min. (P1=0.001)) and aortic cross clamping (exceeding 100 min.) (P2=0.001). Inotrophyc support was administered due to LOS in ten patients (28.5%) from SG, and in 21 patients (60%) in PG, respectively (P<0.001). We used IABP in eight patients in SG (22.8%) and in 17 patients (48.5%) in PG (P=0.0002). Preoperative lower proportion of New York Heart Association and older age, fibrotic LAD artery, and low EF were the main risk factors for perioperative mortality. LAD revascularization decreased the inotrophics and intraaortic balloon pump use both groups (P=0.035). But, numbers of coronary artery anastomosis was not statistically significant role of mortality rates (P=0.089).
Echocardiographic measurements showed that LVEF increased statistically in all survived patients in SG. However, LVEF did not change in 15 patients (42.8%) in PG. Clinical follow-up demonstrated that the left venricular EDVI decreased in all survived patients after surgery in both groups.
The LVEF increased from 34±14 to 41±5 in SG (P=0.001). However, LVEF did not change significantly. The preoperative and postoperative LVEF was 35±16; and 37±3 in, respectively in PG (P>0.05). SVI decreased from 29±10mL/m² preoperatively to 22±14mL/m² in the early postoperative period in PG. This was statistically significant (P=0.027). In SG patients exhibited an increase in SVI after surgery from 33±14mL/m² to 38±5mL/m². This was statistically significant (P<0.001). Table 2 is summarized pre- and postoperative SVI, EDVI, ESVI, LVED and LVESV in both groups in the early postoperative period. The early postoperative data including LOS, mortality rate, intraaortic balloon pump use and arrhytmia have been summarised in Table 3. The changes of left ventricular functions are summarized in Figs. (1 and 2) for SG and PG, respectively.
Shows that the changes of left ventricular functions including postoperative stroke work index, end-systolic volume index, and left ventricular ejection fraction in placebo group. LVEF is stable in 17 patients of placebo group.
Early postoperative data.
| Parameters | LS+SNP(N=50) | PG (n=46) | P value |
|---|---|---|---|
| Hospital mortality* | 2(4%) | 4 (8.6%) | 0.013 |
| Low cardiac output syndrome* | 11 (22%) | 23(36.9%) | 0.036 |
| Intraaortic balloon pumping* | 7 (14%) | 16 (34.7%) | 0.020 |
| Myocardial infarction | 2 (4%) | 7 (15.2%) | 0.024 |
| Atrial fibrillation* | 8 (16%) | 17(36.9%) | 0.001 |
| Complete AV heart block/ | 1 | 2 | 0.610 |
| Reoperation for bleeding | 2 | 4 | 0.420 |
| Renal failure* | 3 (6%) | 12 (27.2%) | 0.002 |
| Pneumonia | 3 (6%) | 3 (6.5%) | 0.870 |
*Statistical significant charecteristic.

Demonstrates that the changes of left ventricular functions including strıke work index and left ventricular ejection fraction in study group of patients. LVEF is increase significantly after operation in number of patients. LVEF shows an increase from 36.7±5.4 to 43±14%. The mean increament of LVEF is 18%± 8 in study group.
Kaplan-Meier Survival Analyses Curve is demonstrated in Fig. (3). During 36 months follow-up, five patients died due to cardiovascular events. Statistical significance has been detected when compared to SVI in the early postoperative period. Post-surgery echocardiographyc examinations showed that there was no any statistical difference between the groups (P=0.687). The left ventricular end-diastolic and end-systolic dimension were also similar in both groups.
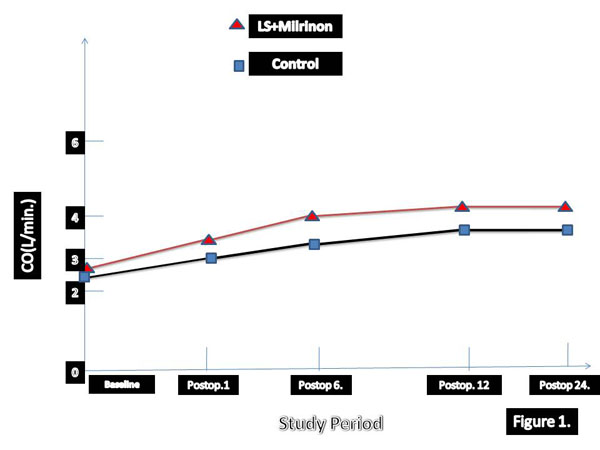
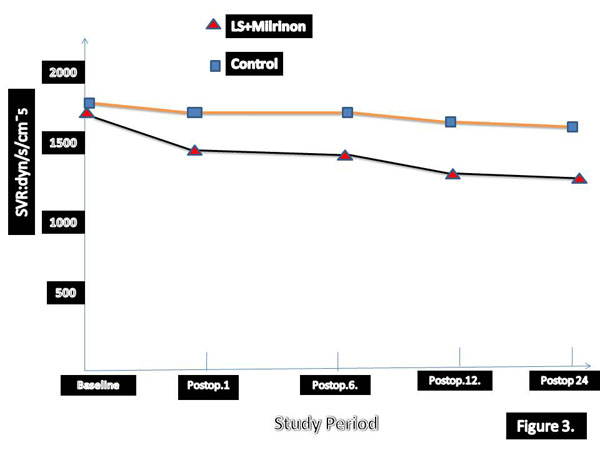
The greater reduction in EDVI and greater increase in EF in early period after surgery have been detected in patients who treated with levosimendan and SNP. According to our results LVEFs were highly correlated to changes in EDVI in SG group. In three times hemodynamic changes has been summarised in Table 4. Univariate and multivariate death from low-output syndrome after surgery has been summarised in Tables 5 and 6, respectively. Long aortic cross-clamp time and concomitant CABG were statistically significant morbidity and mortality factors. We analysed perioperative and postoperative hemodynamic changes over time. Cardiac out-put and CI values were significantly higher in levosimendan group (Table 7).
Hemodynamic changes at three time intervals.
| SVI decreased | SVI increased | P value | |
|---|---|---|---|
| EDVI (mL/m2) | |||
| Pre | 111±33 | 96±25 | 0.02 |
| Early post | 61±10 | 72±11 | 0.001 |
| Late post | 76±19 | 88±18.1 | 0.063 |
| ESVI (mL/m2) | |||
| Pre | 56±27 | 65±19 | 0.624 |
| Early post | 33±8 | 42±8 | 0.075 |
| Late post | 44±7 | 47±17 | 0.080 |
| SVI (mL/m2) | 34±7 | 27±5 | 0.001 |
| Pre | |||
| Early post | 25±6 | 33±7 | 0.001 |
| Late post | 31±4 | 34±5 | 0.675 |
| EF(%) | |||
| Pre | 38±4 | 34±5 | 0.004 |
| Early post | 42±9 | 40±6 | 0.624 |
| Late post | 43±6 | 42±8 | 0.834 |
Exhibites that Kaplan-Meier survival curve (non-adjusted for demographics) for study and placebo group patients. The mortality rate of placebo group is high during observational period (P < 0.01).
Univariate analysis for postoperative low cardiac output syndrome and deaths from congestive heart failure.
| Variable | LCO | Deaths from CHF |
|---|---|---|
| (P value) | (P value) | |
| Preoperative parameters | ||
| Age 65 years | 0.380 | 0.227 |
| Sex | 0.144 | 1.000 |
| Hypertension | 0.470 | 0.725 |
| Diabetes mellitus | 0.469 | 1.000 |
| Impaired renal function | 0.534 | 0.706 |
| Delay from MI2y. | 0.144 | 0.367 |
| NYHA class | 0.190 | 0.154 |
| Angina pectoris | 0.374 | 0.546 |
| Multivessel CAD | 0.512 | 0.115 |
| LVEDP20 mmHg | 0.321 | 0.015 |
| LVEF <40% | 0.580 | 0.538 |
| Operative parameters | ||
| CPB duration>240 min | 0.001 | 0.002 |
| Aortic cross-clamping 120 min | 0.234 | 0.024 |
| Concomitant CABG | 0.037 | 0.465 |
Multivariate analysis for postoperative low cardiac output syndrome and deaths from congestive heart failure.
| Variable | Odds ratio | 95%CI | P value |
|---|---|---|---|
| For early LCO | |||
| Left system coronary artery disease | 6.9 | 1.3-19.6 | 0.024 |
| For death from CHF | |||
| LVEDP >20 mmHg | 7.1 | 1.1-47.6 | 0.028 |
| CI, confidential interval. | |||
Peri- postoperative hemodynamic changes over time.
| 8 h After-CPB | 12hAfter-CPB | 24 h After-CPB | |||
|---|---|---|---|---|---|
| Heart rate | |||||
| Group I | 56 ± 14 | 84 ±6 | 96 ±11 | 90 ±9 | 92 ±7* |
| Placebo | 68 ±18 | 81±7 | 93 ±7 | 97 ± 88 | 89 ±9* |
| Mean Arterial Pressure (mmHg) | |||||
| Group I | 71±8 | 73± 7 | 71±7 | 76 ±13 | 76 ± 10 |
| Placebo | 73±7 | 72±9 | 70 ±9 | 74±9 | 77 ± 12 |
| Pulmonary Capillary Wedge Pressure*(mmHg) | |||||
| Group I | 10±2 | 9±1 | 8±2 | 9±1 | 15 ±2 |
| Placebo | 11±3 | 18±2 | 17±3 | 19±2 | 16 ±2 |
| Central venous presssure (mmHg) | |||||
| Group I | 12 ±3 | 13±3 | 14±3 | 14±3 | 13 ±3 |
| Placebo | 13±2 | 14±2 | 15±3 | 14±4 | 14±2 |
| CardiacOutput (L/min) | |||||
| Group I | 1.8±0.2 | 2.4 ±0.6* | 2.9 ± 0.5* | 3.8 ± 1.6* | 6.4± 0.5*† |
| Placebo | 1.7 ±0.5 | 1.9±0.5* | 1.9 ±0.4* | 2.2 ±0.2* | 4.6 ±0.4* |
| SVR* (dyne/ s/cm-5) | |||||
| Group I | 1644±166 | 883±137* | 792±152* | 788 ±132* | 864 ±142*† |
| Placebo | 1672 ± 155 | 1041± 123* | 1040±140* | 987±162* | 1181 ±182* |
Left Ventricular Size and Functions in Long-Term Follow-Up
In survived patients, echocardiograms were available in the first week post-EVPR, and a median of 6, 4 months (range 3-25 months). Five patients died because of cardiovascular events during the follow-up period (three patients from PG and, two patients from SG). The remaining 60 patients did not differ in clinical and hemodynamic profiles when we compared echocardiographyc controls. Patients in whom SV decreased initially had larger SV at baseline compared with those in whom SV increased initially; they also had larger EDVI and ESVI and greater EF. According to Marui et al., our research showed that the SVI reduction was temporary, and patients who have lower SV showed recovery during the follow-up, so that at the time of chronic follow-up there was no significant difference in SV between the two groups [11, 12]. However, the reduction in EDVI and ESVI persisted along time in the PG. These findings indicate that different baseline loading conditions may affect the response of cardiac function to SVR in SG.
DISCUSSION
Postoperative ventricular failure is a life threatining problem in CABG patients. Impairment of ventricular function produces a reduction in peripheral oxygen transport, which, in turn, leads to a prolonged stay in the ICU. Myocardial β-adrenergic receptor desensitization occurs chronically in patients with congestive heart failure and acutely after ECC, thereby limiting the efficacy of β-adrenergic stimulants for post-bypass cardiac failure. We presented the effects of levosimendan and sodium nitroprusside combination in CABG patients who required EVPR. Our research demonstrated that older age, a low LVEF as independent risk factors. Also, our study was clearly showed that NYHA class III and IV status, longer aortic cross clamp and total ECC time were the most important factors of early mortality in CABG patients who underwent EVPR. To the best of our knowledge, for the first time, we investigated the effects of the use of levosimendan and SNP combination on left ventricular systolic and diastolic functions including SVI, EDVI, ESVI and LVEF in our CABG patients who have LF aneurysm requiring surgical ventricular reconstruction.
Levosimendan is a calcium sensitizers, that increasemyocardial contractility without increasing intracellular calcium, has emerged as an inotropic agent. LS has been used for myocardial preconditioning without the intracellular calcium accumulation during the ischemia-reperfusion injury [13]. Because of adenosine triphosphate (ATP) production, LS provides coronary artery dilationand myocyte mitochondrial activation. These beneficial effects work synergistically with calcium sensitization to ventricular myocardial improvement [13]. Tritapepe et al. reported that levosimendan pre-treatment improves outcomes in patients undergoing CABG patients [14]. Ikonomidis et collegues showed that treatment with LS improved coronary artery flow and microcirculation in parallel with an improvement in cardiac performance and neuro-hormonal activation in patients with advanced heart failure [15]. Levin’s and Malliotakis’s study demonstrated that levosimendan decreased the mortality in high risk patients undergoing CABG with impaired left ventricular function [16, 17]. Our previously published study exhibited that levosimendan decreased atrial fibrillation after CABG [18].
Sodium nitroprusside could potentially influence the left ventricular relaxation by several mechanism in patients with myocardial ischemia [19].
We hypothesised that after EVPR the left ventricular volume may be decreased and diastolic filling rates fell significantly during LV diastole, and did not change and fell significantly, during the last third of diastole. Therefore, these changes in filling rates may contribute to the shifts in the diastolic pressure-volume relation seen in our patients when we administer SNP and levosimendan for ventricular preconditioning. Our study showed that the ventricular diastolic filling rates increased slightly with our pharmacologic approachs in CABG patients who underwent EVPR. The results of our study showed that EF and resting SV was more frequently increased after SVR in SG. In fact, in data of PG cohort showed an average 8 mL/m² (approxymately 14%) decrease in resting SV.
Clinical studies have showed EVPR frequently decreased EDV [17-20]. Vasodilating effects of SNP and its positive impact on afterload after cross clamping increased the LVEF, EDV, and ESV in SG. Our results demonstrated preoperative levosimendan preconditioning and SNP use directly affected on index of LV pump function.
Our study demonstrated that administration of LS combined with SNP increased ventricular relaxation and LVEF after EVPR in dilated ischemic cardiomyopathy. LS and SNP decreased intraaortic balloon pump and inotropic agent requirement. LS and SNP may influence the left ventricular systolic and diastolic pressure-volume relationships. Likewise, the pressure and blood flow in the coronary arteries and their intramural branches could affect the compliance of the left ventricular wall and influence the pressure-volume curve on this basis. Overall, our estimates of mean postoperative SVR changes in SV reported in the literature range from an increase of 15 mL to decreases of 15 mL or greater with, as noted above, a majority of studies showing reductions [21, 22].
Study Limitations
Our data set concerning the impact of EVPR on left ventricular size and function was in the early postoperative period, with a smaller database. Comparison of resting LV size and function between preoperative and early postoperative conditions provides important information. The previous study confirmed that important specific factor was defined as ventricular filling charecteristics and the LV systolic and diastolic properties. The left ventricular clarification such as ventricular diastolic properties associated with better hemodynamic effects of SVR and links between hemodynamic effects and clinical outcomes could help define appropriate patient selection criteria for these pharmacologic approachs and surgical procedures. Despite our limitations, the current results advocate routine use of levosimendan protocol in patients with low LVEF undergoing OPCABG surgery.
CONCLUSION
Preoperative levosimendan and peroperative SNP combined with levosimendan administration in CABG patients with low LVEF undergoing ECC was associated with greater improvement in hemodynamic stability after surgery and early postoperative period. It shows faster onset of action, lower use of inotropic support, and IABP use including shorter ICU and hospital stay and lower rate of complications as well as morbidity. The present study analyzed the early changes in SVI after SVR and attempted to understand the apparent paradox of improved volumes reduction and LVEF with the frequently observed reduction in stroke volume after SVR.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.


